Advances in the Preparation and Application of Nanochitin in the Food Industry
-
摘要: 纳米甲壳素作为一种资源丰富且可再生的生物聚合物,因优异的物理和化学特性在食品领域备受关注。本文首先详述纳米甲壳素的来源、制备方法及其结构特性,重点对近年来国内外关于其在食品活性包装、乳化稳定剂、功能因子载体等方面的应用进行梳理总结。同时借助文献计量学方法深入解析纳米甲壳素在食品领域的研究热点,对其未来的发展方向进行了展望,旨在为纳米甲壳素的综合开发利用提供参考。
-
关键词:
- 纳米甲壳素 /
- 制备方法 /
- 结构性质 /
- 食品领域 /
- CiteSpace可视化分析
Abstract: Nanochitin, a plentiful and renewable biopolymer, has attracted considerable interest in the food industry due to its excellent physical and chemical properties. This paper initially outlines the sources, preparation methods, and structural properties of nanochitin, then focuses on summarizing its recent applications in food active packaging, emulsification stabilizers, functional factor carriers, other fields both domestically and internationally. Additionally, the paper utilizes bibliometric methods to conduct an in-depth analysis of the research hotspots pertaining to nanochitin within the food sector, projecting its future development trajectories. This paper aims to furnish insights for the comprehensive development and utilization of nanochitin. -
甲壳素(Chitin)是仅次纤维素的第二大天然高分子多糖,是由2-乙酰氨基葡萄糖通过β-1,4糖苷键连接而成的线性聚合物多糖,广泛地存在于虾蟹等甲壳动物及乌贼、贝类等软体动物的骨骼中[1−4]。甲壳素具有可再生性[5]、生物相容性[6]、环境友好[7]、生物降解性[8]、无毒[9]、抗菌活性[10]等特性,已被广泛用于乳化稳定剂[11]、食品营养添加剂[12]、活性包装膜[13]和凝胶[14−16]等方面。但是,由于甲壳素分子链间存在大量分子间和分子内氢键相互作用导致其溶解性和加工性差,限制了其在食品领域中的广泛应用。随着纳米技术的发展,其在食品领域中的应用备受关注。纳米甲壳素(Nanochitin,NCh)是一类用物理或化学方法打破甲壳素的长链结构而形成的纳米级甲壳素材料。NCh不仅具有甲壳素的性质,而且能够在水溶液中形成均匀的分散液,同时还具有纳米材料的特性,包括小尺寸、低密度、高表面积、优异的生物相容性和力学性能、可再生性等[17],已被研究应用于复合材料[18]、医药[19]和食品[20]等领域。同时,NCh表面含有羟基、氨基等活性官能团易于进行化学修饰,从而进一步提高NCh的应用潜力。不同的制备方式对NCh的形态特征、物理化学性质产生显著的影响,使其呈现出不同的特性。根据结构和形态可以将NCh分成甲壳素纳米晶须(Chitin nano-whiskers,CNWs)和甲壳素纳米纤维(Chitin nanofibers,ChNFs)两大类。在食品领域中,NCh因其优越的力学性能、阻隔性、两亲性和多羟基官能团等特点[21],使其在新型食品活性包装、乳化稳定剂、功能因子载体、品质改良剂等方面的应用受到广泛的关注。
本文对近年国内外NCh在食品领域方面的研究进行梳理,重点阐述其在新型食品活性包装、乳化稳定剂、食品功能因子递送体系等方面的应用进展,结合CiteSpace可视化分析当前NCh领域的研究热点,同时对NCh未来发展进行展望,以期为NCh的进一步应用研究提供参考。
1. 纳米甲壳素的分类及制备方法
1.1 纳米甲壳素的分类
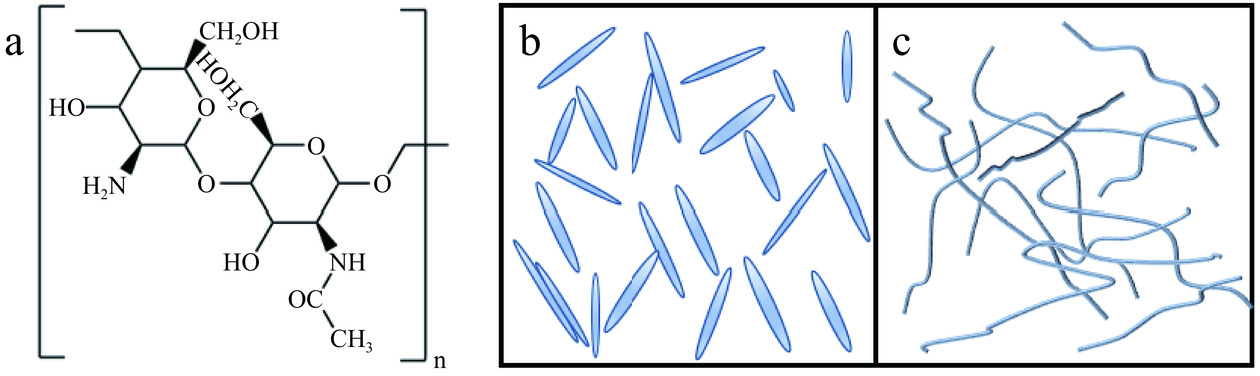
纳米甲壳素根据结构和形态可以分成甲壳素纳米晶须(Chitin nano-whiskers,CNWs)和甲壳素纳米纤维(Chitin nanofibers,ChNFs),NCh的结构与分类的外貌形态如图1所示。
CNWs是通过酸解工艺除去甲壳素纤维中无序部分而形成具有高结晶度的棒状或针状纳米晶体,通常长度在150~2200 nm之间,宽度为10~50 nm,长径比在5.0~122.2之间[22]。CNWs含有丰富的活性基团,采用酸解法可使其表面富含羟基、乙酰氨基,还有少量的氨基[23],氨基的存在可使甲壳素表面带正电。CNWs具有高的比表面积[24]、良好的机械强度和优异的生物相容性[25]。
ChNFs主要是通过机械处理获得的一种纳米纤维素类型,但由于机械处理的局限性,ChNFs中仍然存在非结晶区,相对于CNWs的结晶度较低,尺寸更大[26]。由甲壳素分子有序堆积而成的ChNFs,具备甲壳素所有良好的性能,如可生物降解性[8]、生物相容性[27]、独特的抗菌性[10]和优良的吸湿、透湿性[28]等。由于ChNFs原子排列高度有序,而且直径极小,不含普通材料所具有的晶界、位错、空穴等缺陷,因而具有高强高模的特点,是一种性能优异、环境友好、天然的增强材料[26]。
1.2 纳米甲壳素的制备方法
甲壳素作为重要的海洋废弃物综合利用加工产物,是一类环境友好型绿色可再生资源[30]。制备纳米甲壳素的一般流程是首先从天然生物组织中提取粗甲壳素粉,再将粗甲壳素粉进行提纯,即去除蛋白质、矿物质、脂质以及色素等,并以一定的物理化学方法将甲壳素纳米化,从而制备出纳米甲壳素[31−33]。
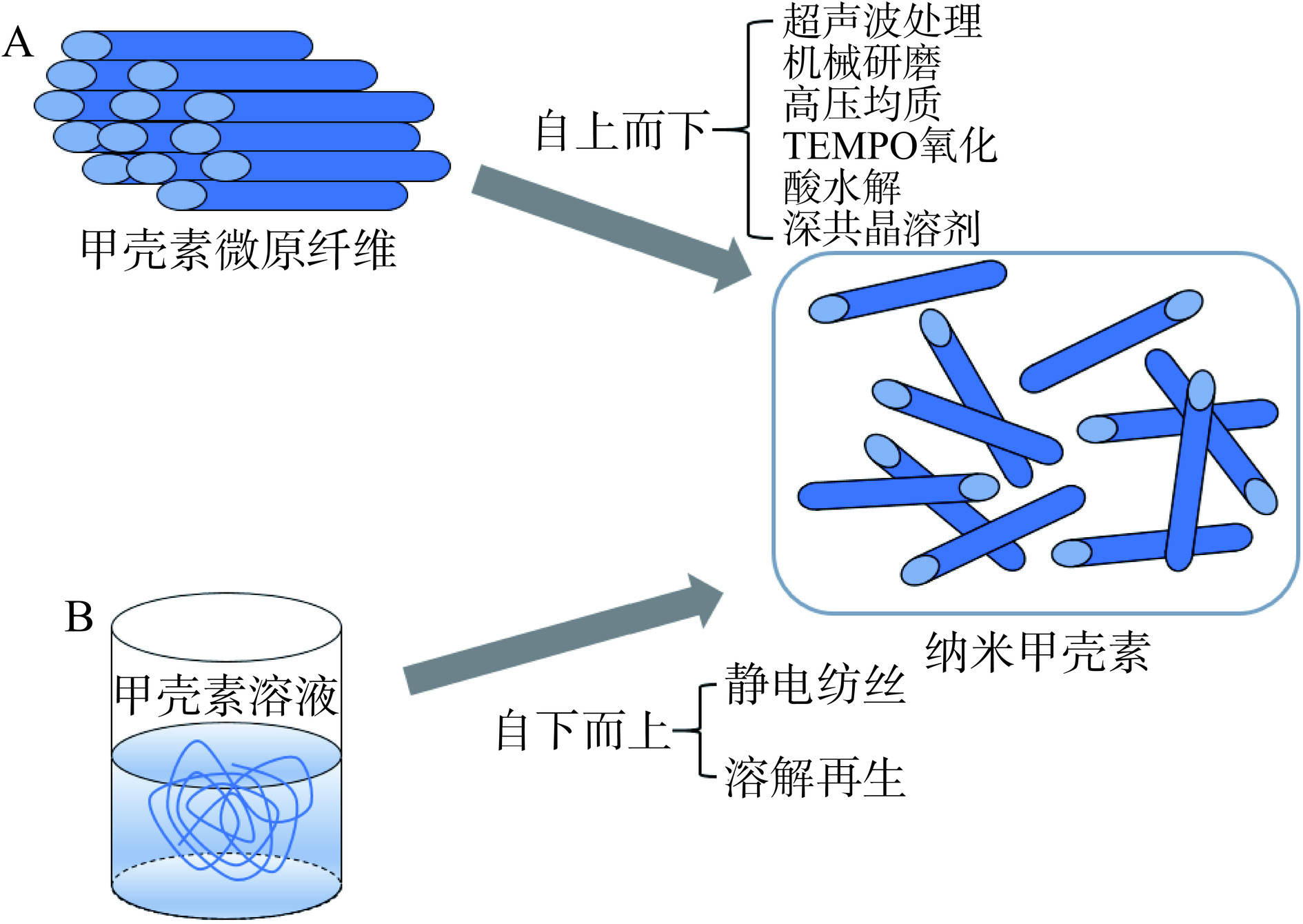
根据生物纤维材料的制备工艺的不同,可将NCh的制备方法概括为两大类:“自上而下”(“Top-Down”)和“自下而上”(“Bottom-Up”)[34],如图2所示。“自上而下”的制备原理通常是利用伯胺质子化和强烈的机械作用,将大分子的甲壳素通过外力作用制备产生纳米级的ChNFs,但不会破坏分子长链[29,32,35]。而“自下而上”的制备原理则是在甲壳素溶液加工过程中破坏强氢键作用,可将分子链破坏,使其完全溶解,再通过一定的条件将溶液中的小分子甲壳素自组装为纳米级的甲壳素[36]。近年来,采用有机溶剂将甲壳素完全溶解为小分子后,通过静电纺丝和溶解再生组装为纳米纤维的方法也备受关注[37]。采用“自上而下”的传统纳米化的制备方法更加广泛,包括机械处理法、TEMPO氧化法等。
1.2.1 基于“自上而下”的纳米甲壳素的制备方法
在传统的“自上而下”制备方法中,机械处理方法制备原理简单、操作便利、安全无毒、对于产品的尺寸也可通过处理工艺进行控制,因此是运用最广泛的方法[38]。酸碱提纯后的纯甲壳素在酸水解反应下非结晶区将被裂解,同时甲壳素的伯胺基团在酸溶液中被质子化产生阳离子,强烈的氢键作用被阳离子产生的静电相互作用破坏,从而获得更小的纳米纤维束,再利用强机械力将紧密的甲壳素纤维束分裂开,从而制备产生更小的纳米甲壳素[39−41]。目前,有大量文献报道了利用不同机械力来制备纳米级甲壳素纤维,比如超声波、研磨机、高压均质等。
1.2.1.1 超声波处理法
近年来,超声波处理法被广泛应用于纳米材料的合成,尤其是在基质包裹纳米纤维的组合体生物资源中,主要原因在于超声波所产生的空穴效应,它能通过强大的拉应力使得液体产生局部的高温、高压环境,破坏分子间氢键作用力,从而将结合紧密的甲壳素纤维束分裂,产生更细小的纳米纤维结构[42]。Lu等[43]以虾壳为原料,在中性条件下采用高强度超声处理可将天然甲壳素分解成宽度均匀的高纵横比的ChNFs。同时通过调节超声时间,可实现不同粒径的ChNFs的可控制备(20~200 nm间)。Hsueh等[42]以β-甲壳素为原料,在冰水浴条件下,使用超声处理器分别超声1和2 h,结果发现超声1 h的纳米纤维表现出柔韧的毛状纤维外观,平均直径为17.24 nm,纤维长度为1725.05 nm,长径比为100:35,而延长处理后的纳米纤维平均直径为15.67 nm。
1.2.1.2 研磨法
研磨法通常是借助超微粉碎机的强大机械力将甲壳素悬浮液中的甲壳素纤维束打开,从而获得更小的单体纤维,并且能够依据磨盘种类、磨盘间隙与回转数来调整纤维尺寸大小[44−45]。Ifuku等[44]以干蟹壳为原料,去除蛋白质和矿物质后,溶解于醋酸水溶液中,通过研磨机研磨之后,得到的晶体状的ChNFs具有均匀的宽度(约为10~20 nm)和高长径比。同时,表征结果显示N-乙酰基没有被去除,α-甲壳素晶体结构也保持不变,进一步证实了纳米纤维是从天然甲壳素/蛋白质/矿物复合材料的原始状态提取的。Siahkamari等[46]将甲壳素微纤维原粉制备成浓度为1%wt的悬浮液,搅拌均匀后倒入机器中进行循环研磨。经过剪切力和压缩力的作用,原纤维束发生了纳米级原纤化过程,从而产生的ChNFs呈网络形状,其中大多数ChNFs的直径尺寸约为60 nm[32]。Fazli等[47]摒弃传统酸处理脱盐的方法,以一种基于热水、碱液提取和低能量混合的温和提取法以及简单机械研磨从蘑菇干粉中制备了ChNFs,同时保留了大量葡聚糖(50%~65%),该研究表明葡聚糖的存在能够大大提高ChNFs的机械性能,强度是现已报道的ChNFs的最高值。
1.2.1.3 高压均质法
高压均质的原理是对于注入高压均质腔内的具有一定的流速的液体悬浮液,在腔室循环的过程中,受到高压剪切力、对流碰撞力等机械力作用,使得悬浮液的物质细化,以达到均质的效果[48−49]。Gao等[40]通过机械高压均质的方法分别处理了添加与未添加壳聚糖的甲壳素悬浮液,成功制备了ChNFs。表征结果显示在不添加壳聚糖的情况下,通过机械均质可以得到宽度约为4.6 nm的ChNFs。但是,样品中也发现了较大的ChNFs或甲壳素/蛋白质束的聚集物,其宽度约为20~100 nm;而对比在pH3的高压均质过程中,壳聚糖的加入显著降低了ChNFs的团聚,均质后的ChNFs主要呈现单体约为4.6 nm等宽的纤维,未见较大直径的纤维。研究表明在机械均质过程中适量添加壳聚糖能够促进甲壳素的原纤化过程,防止甲壳素聚集。Salaberria等[48]提出动态高压均质这一概念,通过对流体施加非常高的压力,将分散的颗粒细分成非常小的尺寸,甚至达到纳米级别。利用动态高压均质的方法,以黄龙虾废弃物为原料,成功地制备了直径100 nm以下的均匀的ChNFs。
1.2.1.4 TEMPO氧化法
TEMPO氧化法是通过TEMPO/NaBr/NaClO氧化体系对甲壳素中的C6位羟甲基进行选择性氧化,然后结合机械处理的方式可以制备出羧基化的纳米甲壳素[50−51]。Ye等[52]采用机械粉碎结合TEMPO介导氧化的方法,制备出了氧化纳米纤维,研究表明先采用水相碰撞粉碎甲壳素胶束可以提高氧化效率,在所需的氧化剂量相同的条件下,氧化后的ACC-纳米纤维的羧酸含量比报道的直接TEMPO氧化法增加了一倍。Ye等[53]分别采用TEMPO/NaClO/NaClO2以及TEMPO/NaBr/NaClO两种不同氧化体系制备了尺寸存在差异的氧化NCh,前者制备出平均宽度为16.67±7.9 nm,平均长度为770±170 nm的纤维,后者制备的纤维宽度大多为20~24 nm,平均长度为1 μm,表明不同氧化过程对纳米纤维材料产生了尺寸效应。
1.2.1.5 酸水解
在酸水解过程中,甲壳素的无序和弱横向结构部分优先被水解并溶解在酸溶液中,而不溶于水的、具有较强耐酸性的高结晶残基则保持完整[45]。因此,NCh是酸水解的结果,它消除了无序和低横向有序的晶体缺陷[54]。用3 mol/L HCl在煮沸(104 ℃)条件下水解纯甲壳素90 min;酸水解后用去离子水稀释悬浮液,离心,取上清;这个过程重复多次,直到悬浮液自发地变成胶体。所形成的晶体为棒状颗粒,平均长200±20 nm,宽8±1 nm,可浓缩成液晶相,在一定浓度下可自组装成液晶相[27]。除了在HCl溶液中水解外,H2SO4也被用于制备纳米甲壳素[55−56]。因强酸运输、设备和成本高以及污染环境等问题阻碍了使用强酸处理的纳米甲壳素的商业化和工业化[57]。近年来,一直都在尝试用便捷环保的方法制备甲壳素纳米纤维。Liu等[58]利用固体马来酸水解从贝壳中分离出NCh,所得的NCh为棒状,产率为1.59%~10.42%,粒径为397.8~170.6 nm,表面羧基含量为0.04~0.173 mmol/g。与HCl和H2SO4相比,马来酸具有高存储安全性、低运输成本、环境友好性和最小的设备腐蚀、更高的沸点等优点。
1.2.1.6 深共晶溶剂(DES)
DES是一种绿色溶剂,由氢键供体(HBD)和氢键受体(HBA)在特定温度下组合而成,具有良好的溶剂容量和低蒸汽压[59],同时能够有效地与碳水化合物和生物质的氢键系统相互作用,因此已被认为是多糖绿色加工的可持续和可配置的化学物质[60]。Mushi等[61]进一步研究发现,DESs(氯化胆碱-硫脲比例1:2)可以制备宽25~45 nm,长162~450 nm的ChNFs。同样,使用氯化胆碱和五种不同有机酸(乳酸、草酸、柠檬酸、丙二酸和dl-苹果酸)制备的酸性DESs,生产收率高达100% CNWs[62]。
1.2.2 基于“自下而上”的纳米甲壳素制备方法
静电纺丝法是指能够在高压电场的作用下,利用高分子材料制备出连续纳米纤维的技术,当电场力足以克服注射器尖端液滴的表面张力时,带电聚合物射流就会从泰勒锥中喷射出来,然后以膨胀螺旋的形式拉长,获得纳米纤维[63−64]。Shamshina等[65]将虾壳废弃物溶解在离子溶液中,再通过纯化处理去除蛋白等杂质后,直接采用静电纺丝技术制备ChNFs,结果表明整个制备流程简单、成本低,并能制备出性能良好的纳米纤维。溶解再生法是将甲壳素先溶解在特殊的溶剂中,然后通过加入其他试剂诱导再生出NCh的方法[66−67]。
超声、研磨等机械处理法是通过物理的手段对甲壳素进行处理,不能完全地将甲壳素纳米纤维化,会存在一定数量的纤维束或未纤维化部分,但是具有生产能力高、污染小等优点,有利于工业化。TEMPO氧化法和酸水解法所制备的机理都是靠静电斥力破坏甲壳素分子间的氢键,TEMPO氧化法是靠羧基阴离子,而酸水解法是胺基阳离子。由于TEMPO 氧化法会产生更多静电斥力,因此这种方法基本能完全使甲壳素纳米化,而酸水解法能够观察到聚集现象,只能部分使甲壳素纳米化,为了提高纳米化程度,酸水解后需要进行超声或其它机械处理,同时还存在产率低的缺点。深共晶溶剂能代替对环境有害的化学试剂,同时还具有热稳定性好、价格低、毒性低和可制定性的优点,但由于其极强的亲水性会降低NCh的产率。静电纺丝法需要对甲壳素降解,采用的溶剂大多有毒。溶解再生法制备NCh不需要特殊的化学设备,也不需要对甲壳素进行化学改性,同时这些溶剂可以回收重复使用,但是成本高,不利于工业化。
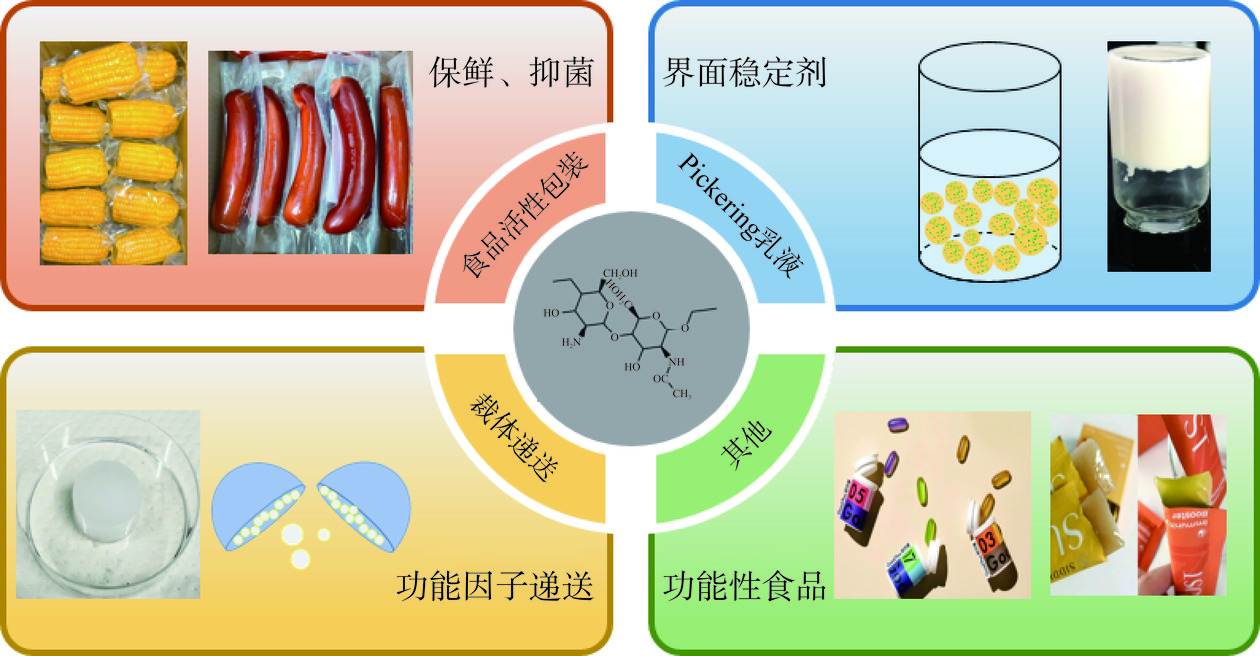
2. 纳米甲壳素在食品领域中的应用
2.1 作为生物活性材料用于食品活性包装材料
NCh具有较高的机械强度、较大长径比、抗菌活性、生物可降解及天然无毒等特点,可以作为食品包装中最为广泛的生物活性材料(图3)。Wu等[68]将天然红甘蓝提取物和乳酸链球菌素固定在用ChNFs增强的普鲁兰多糖/壳聚糖复合基质上制备了一种可动态监测食品新鲜度的新型多功能纳米复合膜。Jiang等[69]将魔芋葡甘聚糖和ChNFs与柠檬酸结合制备得到具有优良的机械强度和抗菌活性的生物纳米复合薄膜。Mushi等[61]在壳聚糖中加入体积分数为8%的纳米甲壳素使其复合材料表现出模量、强度和应变破坏的独特组合,同时也使得断裂功率高达35 mJ/m3。Duan等[70]报道了一种可作食品包装应用的含有姜黄素和花青素的普鲁兰/ChNFs的静电纺纳米纤维,发现纳米纤维可作为监测食品品质变化的pH指示剂。另外,Salaberria 等[71]在聚乳酸中加入CNWs制备了可持续包装的功能性纳米薄膜,这种薄膜对霉菌黑曲霉等微生物具有良好的抑制作用。Xie 等[72]将ChNFs与葱花提取物结合制备的复合薄膜能有效地保存新鲜切好的香蕉,同时,与阿魏酸复合涂层结合的ChNFs能通过降低呼吸和水分流失以及抑制微生物生长来延长草莓和鲜切苹果的保质期。
2.2 作为界面稳定剂用于Pickering乳液
Pickering乳液是以超细固体颗粒作为乳化剂而得到的稳定的乳液。由于Pickering乳液能被糖、蛋白质等固体颗粒所稳定,而且具有良好的结构稳定性和生物相容性,在食品领域得到广泛应用[73]。NCh因其具有足够的界面活性和天然安全等特性,常作为稳定剂加入到食品级Pickering乳液中[11]。Sun等[74]将ChNFs加入到胶体玉米蛋白形成的Pickering乳液中,提高了乳液的稳定性和功能特性。另一方面,油脂含量较多的食品在贮藏期间容易发生油脂氧化,导致营养成分损失,而通过NCh稳定的Pickering乳液能延缓油脂氧化。Wang等[75]制备得到含有NCh和单宁酸复合的Pickering乳液,发现添加0.3%NCh和0.2%单宁酸所形成的复合物能有效延缓乳液中的油脂氧化。Zhong等[76]采用ChNFs制备含有柑橘精油的Pickering 乳液,发现ChNFs能有效稳定CEO Pickering 乳液并使其具有pH可逆响应特性及提高了CEO的抗菌活性。
2.3 作为载体应用于食品功能因子递送体系
基于NCh的纳米粒子[77]、凝胶[78]和Pickering乳液[79]等载体在封装食品功能因子方面的研发应用逐渐增多。Dhanasekaran 等[77]采用NCh作为纳米粒子递送姜黄素和胰岛素,实现了姜黄素和胰岛素的高效包封和缓释。Petrova等[78]用部分脱乙酰化的CNWs填充藻酸盐水凝胶,用于四环素的封装与缓释,延长了四环素在体内释放的时间。Jia等[79]用TEMPO氧化的CNWs稳定Pickering 乳液,用于槲皮素封装与递送,实现了对槲皮素的释放有效控制。Torlopov等[80]采用NCh稳定的橄榄油乳液递送维生素D3,改善了维生素D3在小肠中的释放效果。
2.4 其他食品方面应用
NCh对人体健康有众多有益作用,包括增强免疫力、调节胃肠道、减肥和减脂,以及添加到食品中的NCh可以赋予特定的功能作用。因此,NCh 在人类健康或功能性食品领域将具有许多应用前景。Ngasotter等[27]发现NCh可以促进肠胃蠕动并稳定肠道菌群,从而提高人体免疫力。Zou 等[81]发现ChNFs可用于阻碍脂质消化,促进饱腹感和减少热量摄入,改善健康,在一定程度上能起到“减肥”功效。同时,也有研究发现在食品乳液中加入NCh,可以增强胃饱腹感、调节脂质消化和控制热量摄入[82]。由NCh稳定的高内相乳液成为可行的脂肪代替品,此外,高内相乳液的应用已经拓展到多功能食品油墨的开发,特别是在食品3D打印领域[83]。Zhu等[84]通过脱乙酰化甲壳素的表面阳离子化制备了 ChNFs,有效地稳定了含有88%葵花籽油、0.5%wt浓度的高内相乳液,并且成功地通过墨水直接进行3D打印,可用于可食用的功能性食品和显示高度互连的轻孔固体泡沫。这些研究不仅强调了NCh增强乳液稳定性的能力,而且促进了其作为食品3D打印的新型生物油墨材料的有前途的用途。
此外,NCh也可以作为食品中的功能性成分。CNWs具有作为一种新型的老化抑制剂的潜在性,可能用于生产具有较长保质期的淀粉基产品。有研究报道称,在普通玉米淀粉、糯玉米淀粉和甘薯淀粉中添加10%wt CNWs可以抑制淀粉的老化[85]。目前,一些研究员已研究NCh作为潜在的增盐剂,当pH<7时,ChNFs分子链上的氨基团发生质子化,使其带正电荷。当氯化钠加入到ChNFs溶液中,会被解离成Na+和Cl−,ChNFs表面的NH3+与Cl−结合形成双电层,增加溶液中游离的Na+。游离的Na+的增加会通过促进Na+和味觉受体之间的相互作用来增强对咸味的感知[86]。
3. 基于CiteSpace的纳米甲壳素研究形态分析
收集2005~2024年期间 Web of Science (WOS) 数据库中有关NCh的研究文献进行关键词分析,并深入反映文献研究内容和该领域的研究热点。
WOS数据库检索检索式:TS=(nanochitin OR chitin nanofibers OR chitin nanowhisker)and food time sapn=2005~2024 索引=SCI-EXPANDED and SSCI,论文类型选择Article OR Review,语种选择English。除去重复的论文,共检索到369篇论文,将其导入CiteSpace软件对关键词进行可视化分析。
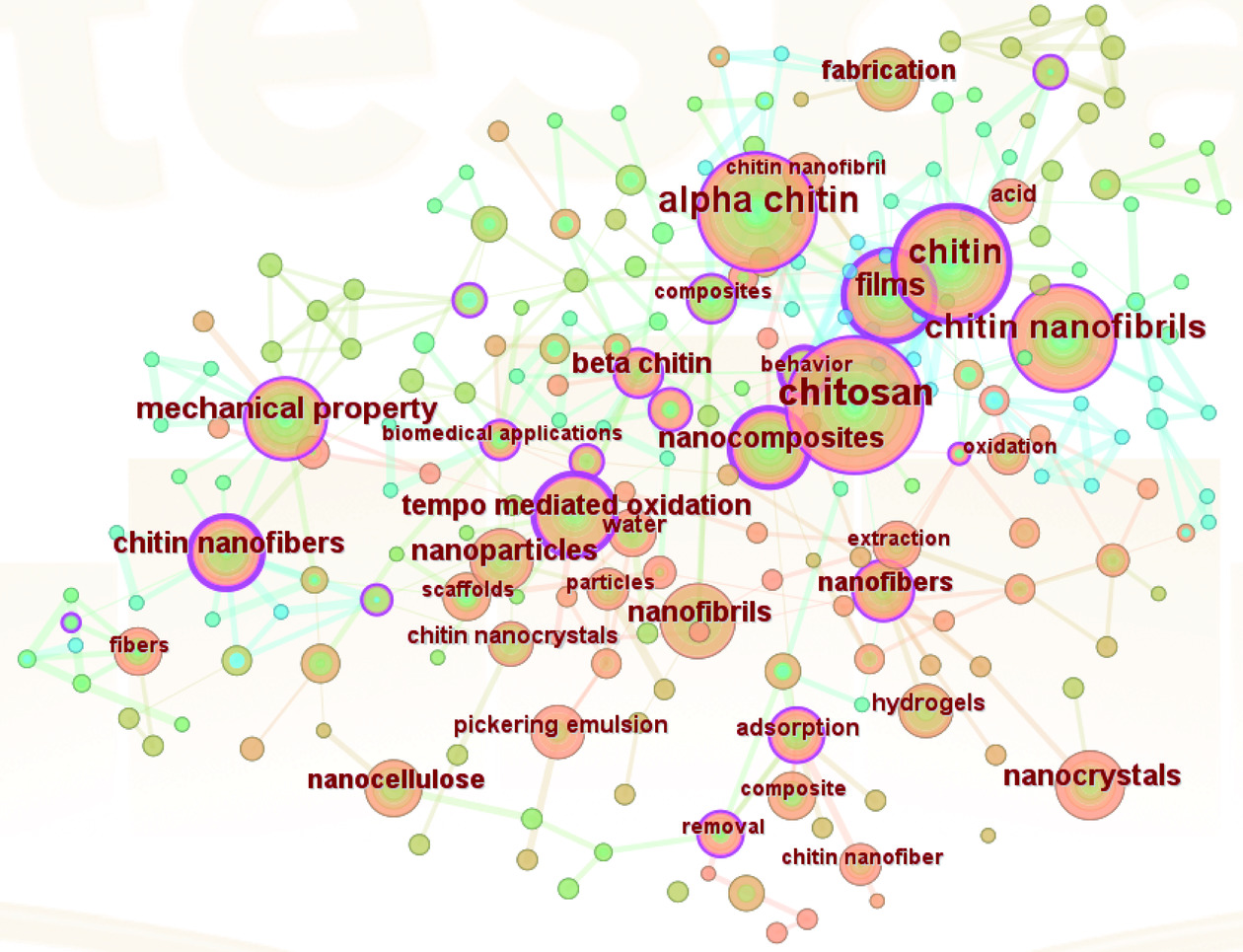
应用CiteSpace对关键词进行共现分析 (图4),图中共有201个关键词构成的关联节点,节点数量较多,说明该领域研究类型比较广泛,研究机制较为复杂。节点越大,表示该关键词在文献中出现的频次越多。由图可知,出现频次前10的关键词分别为壳聚糖、α-甲壳素、甲壳素纳米纤维、薄膜、甲壳素纳米晶须、纤维素纳米晶、β-甲壳素、TEMPO氧化、抗氧化、高压均质、深共晶溶剂和食品包装。其中“chitosan(壳聚糖)”“alpha chitin(α-甲壳素)”“chitin nanofibers(甲壳素纳米纤维)”等关键词是研究的支撑点,也反映了在食品领域的研究热点。
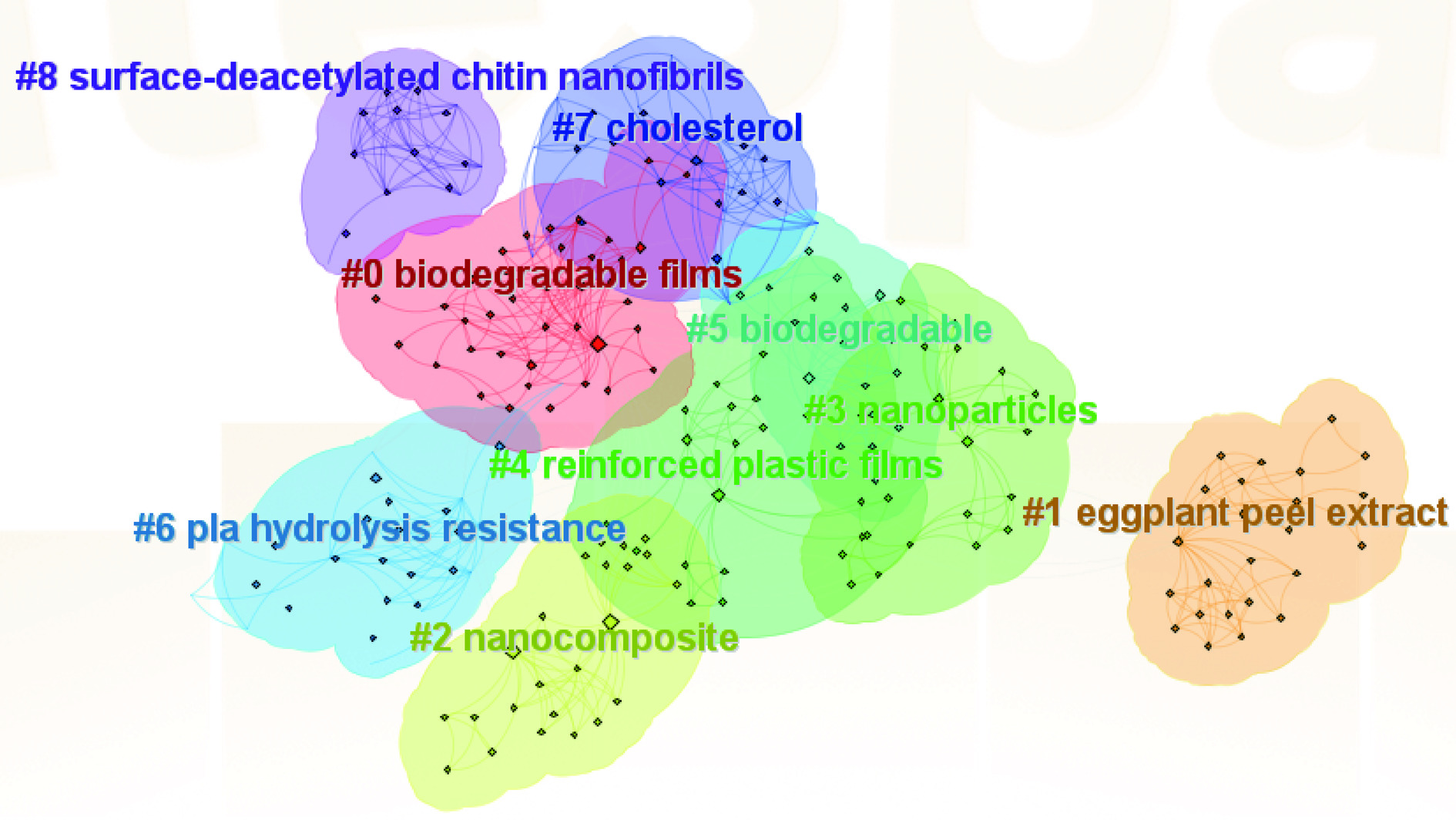
关键词聚类分析是将相似的关键词放在一起,可以明确看清网络关系图被分为几类。应用CiteSpace 的对数似然比算(LLR)对关系紧密的关键词进行聚类分析,并提取相关术语进行聚类命名,得到关键词聚类图[87]。当聚类模块值Q值>0.3表示聚类结构显著,平均轮廓值S值>0.5表示聚类合理[88]。在关键词共现的基础上进行聚类分析,WOS可视化显示Q=0.7478,S=0.9176,此聚类具有一定的显著性和可信性,聚类从0开始编号,即聚类#0是最大的集群,聚类#1是第二大的,依此类推。如图5所示,WOS中纳米甲壳素研究的关键词可聚为9类,分别为biodegradable films(可生物降解薄膜)、eggplant peel extract(茄子皮提取)、nanocomposite(纳米复合物)、nanoparticles(纳米颗粒)、reinforces plastic films(增强塑料薄膜)、biodegradable(可生物降解)等主题为研究热点。其中chitin(甲壳素)、chitin nanofiber(甲壳素纳米纤维)、chitin nanowhiskers (甲壳素纳米晶须)等关键词强调了甲壳素和NCh是食品领域中所研究的热点材料。
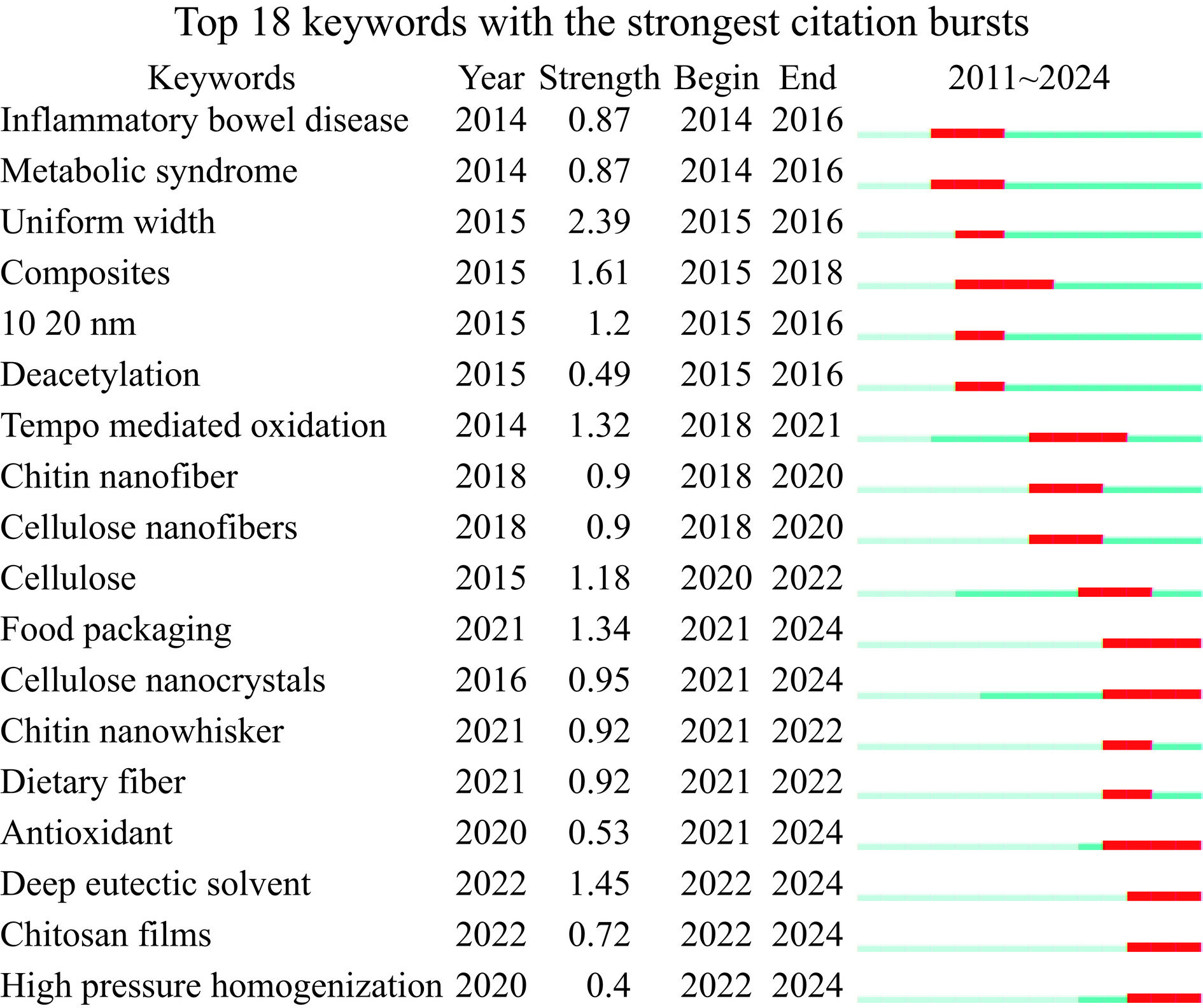
关键词突现分析能够展现短时间内关键词的变化情况,把握不同时期各个阶段的研究热点,反映该主题研究的前沿议题。因此,通过分析NCh相关文献关键词的突现情况,可以辨别和探测该主题的研究前沿主题,为之后的研究提供一些可能的发展方向。如图6所示,检索出突变词一共有18个,突变词的时间跨度不一,最短为1年,最长为3年。2014~2018年为早期前沿阶段,研究内容包括Inflammatory bowel disease(炎症性肠病)、Metabolic syndrome(代谢综合征)、Composites(复合物)、Deacetylation(脱乙酰)等;2018~2022年为中期前沿阶段,研究内容包括Tempo mediated oxidation chitinnanocrystals(TEMPO氧化甲壳素纳米晶)、Chitin nanofiber(甲壳素纳米纤维)等;2022~2024年为近期前沿阶段,主要的研究内容为Food packaging(食品包装)、Emulsion(乳液)、Antioxidant(抗氧化)、Chitosan films(壳聚糖薄膜)等。这些突现词反映了近几年NCh研究的前沿热点。
4. 结语
纳米甲壳素(NCh)在食品领域中具有可持续应用的巨大潜力,但NCh的制备以及在食品中的应用研究仍在探索中。文中所讨论的NCh可以采用“自上而下”和"自下而上"的方法从天然甲壳素中所提取,但这些方法大多数依赖于化学品及能源的消耗,容易对环境造成负面影响,而且成本高。现阶段NCh的制备仍处于实验室研究,各种制备方式都具有一定的优缺点,仍然无法进行大规模的生产,原因包括搭建合适的生产环境需要极其精细的条件,而且可能存在产量低等缺陷。因此,在开发新的简单有效的成本合理的制备方法来分离NCh方面仍有大量的研究需要探索。该综述还讨论了NCh作为生物活性材料用于食品活性包装材料、Pickering 乳液的稳定剂、功能性食品中的主要成分和食品功能因子递送载体。同时借助文献计量学的方法,对NCh的研究文献进行数据可视化分析,结果显示NCh、CNWs 和ChNFs等是食品领域中所研究的热点材料,近几年的研究热点包括食品包装、纤维素纳米晶、抗氧化、壳聚糖薄膜等,为NCh在食品领域中的进一步开发利用提供参考。
-
-
[1] WU J, LIN H, MEREDITH J C. Poly(ethylene oxide) bionanocomposites reinforced with chitin nanofiber networks[J]. Polymer,2016(84):267−274.
[2] SALABERRIA A M, LABIDI J, FERNANDES S C M. Different routes to turn chitin into stunning nano-objects[J]. European Polymer Journal,2015,68:503−515. doi: 10.1016/j.eurpolymj.2015.03.005
[3] ISLAM S, BHUIYAN M A R, ISLAM M N. Chitin and chitosan:Structure, properties and applications in biomedical engineering[J]. Journal of Polymers and the Environment,2016,25(3):854−866.
[4] MOHAN K, RAVICHANDRAN S, MURALISANKAR T, et al. Extraction and characterization of chitin from sea snail conus inscriptus (Reeve, 1843)[J]. International Journal of Biological Macromolecules,2019,126:555−560. doi: 10.1016/j.ijbiomac.2018.12.241
[5] XU J, LIU S, CHEN G, et al. Engineering biocompatible hydrogels from bicomponent natural nanofibers for anticancer drug delivery[J]. J Agric Food Chem,2018,66(4):935−942. doi: 10.1021/acs.jafc.7b04210
[6] XU J, DENG X, DONG Y, et al. High-strength, transparent and superhydrophobic nanocellulose/nanochitin membranes fabricated via crosslinking of nanofibers and coating F-SiO2 suspensions[J]. [J]. Carbohydrate Polymers,2020,247:116694. doi: 10.1016/j.carbpol.2020.116694
[7] YOUNES I, HAJJI S, RINAUDO M, et al. Optimization of proteins and minerals removal from shrimp shells to produce highly acetylated chitin[J]. International Journal of Biological Macromolecules,2016,84:246−253. doi: 10.1016/j.ijbiomac.2015.08.034
[8] USMAN A, ZIA K M, ZUBER M, et al. Chitin and chitosan based polyurethanes:A review of recent advances and prospective biomedical applications[J]. International Journal of Biological Macromolecules,2016,86:630−645. doi: 10.1016/j.ijbiomac.2016.02.004
[9] ZHANG J, MOHD SAID F, JING Z. Hydrogels based on seafood chitin:From extraction to the development[J]. International Journal of Biological Macromolecules,2023,253:126482. doi: 10.1016/j.ijbiomac.2023.126482
[10] ZHANG H, XU M, LUO H, et al. Interfacial assembly of chitin/Mn3O4 composite hydrogels as photothermal antibacterial platform for infected wound healing[J]. International Journal of Biological Macromolecules,2023,243:124362. doi: 10.1016/j.ijbiomac.2023.124362
[11] LÜ S, ZHOU H, BAI L, et al. Development of food-grade pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin[J]. Food Hydrocolloids,2021,113:106451. doi: 10.1016/j.foodhyd.2020.106451
[12] WANG M, ZHOU J, SELMA-ROYO M, et al. Potential benefits of high-added-value compounds from aquaculture and fish side streams on human gut microbiota[J]. Trends in Food Science & Technology,2021,112:484−494.
[13] WENG S, MARCET I, RENDUELES M, et al. Insect-derived materials for food packaging-a review[J]. Food Packaging and Shelf Life,2023,38:101097. doi: 10.1016/j.fpsl.2023.101097
[14] DUAN B, LIU F, HE M, et al. Ag-Fe3O4 nanocomposites@chitin microspheres constructed by in situ one-pot synthesis for rapid hydrogenation catalysis[J]. Green Chem,2014,16(5):2835−2845. doi: 10.1039/C3GC42637H
[15] KING C, SHAMSHINA J L, GURAU G, et al. A platform for more sustainable chitin films from an ionic liquid process[J]. Green Chemistry,2017,19(1):117−126. doi: 10.1039/C6GC02201D
[16] HUANG J, ZHONG Y, ZHANG L, et al. Extremely strong and transparent chitin films:A high-efficiency, energy-saving, and "Green" route using an aqueous KOH/Urea solution[J]. Advanced Functional Materials,2017,27(26):1701100. doi: 10.1002/adfm.201701100
[17] JAHED E, KHALEDABAD M A, ALMASI H, et al. Physicochemical properties of carum copticum essential oil loaded chitosan films containing organic nanoreinforcements[J]. Carbohydrate Polymers,2017,164:325−338. doi: 10.1016/j.carbpol.2017.02.022
[18] 李彩荣. 改性甲壳素晶须/聚乳酸纳米复合材料的制备及其性能研究[D]. 广州:暨南大学, 2015. [LI C R. Preparation and properties of modified chitin whisker/ PLLA nanocomposites[D]. Guangzhou:Jinan University, 2015.] LI C R. Preparation and properties of modified chitin whisker/ PLLA nanocomposites[D]. Guangzhou: Jinan University, 2015.
[19] 吴双泉. 甲壳素/碳纳米管复合材料的构建及其在生物医学的应用[D]. 武汉:武汉大学, 2017. [WU S Q. Construction and biomedical applications of chitin/carbon nanotube composite materials[D]. Wuhan:Wuhan University, 2017.] WU S Q. Construction and biomedical applications of chitin/carbon nanotube composite materials[D]. Wuhan: Wuhan University, 2017.
[20] ZHOU H, TAN Y, LÜ S, et al. Nanochitin-stabilized pickering emulsions:Influence of nanochitin on lipid digestibility and vitamin bioaccessibility[J]. Food Hydrocolloids,2020,106:105878. doi: 10.1016/j.foodhyd.2020.105878
[21] ZOU Y, LI X, YU J, et al. The effect of nanochitin on gastrointestinal digestion of starch and protein:Role of surface charge and size[J]. Food Hydrocolloids,2024,146:109312. doi: 10.1016/j.foodhyd.2023.109312
[22] 欧贤凤. 甲壳素纳米晶的改性及其用于染料吸附和双疏涂层的研究[D]. 广州:暨南大学, 2020. [OU X F. Modification of chitin nanocrystals and their application in dye adsorption and amphiphobic coating[D]. Guangzhou:Jinan University, 2020.] OU X F. Modification of chitin nanocrystals and their application in dye adsorption and amphiphobic coating[D]. Guangzhou: Jinan University, 2020.
[23] ARAKI J, KURIHARA M. Preparation of sterically stabilized chitin nanowhisker dispersions by grafting of poly(ethylene glycol) and evaluation of their dispersion stability[J]. Biomacromolecules,2014,16(1):379−388.
[24] GOODRICH J D, WINTER W T. r-Chitin nanocrystals prepared from shrimp shells and their specific surface area measurement[J]. Biomacromolecules,2007,8(1):252−257. doi: 10.1021/bm0603589
[25] WONGPANIT P, SANCHAVANAKIT N, PAVASANT P, et al. Preparation and characterization of chitin whisker-reinforced silk fibroin nanocomposite sponges[J]. European Polymer Journal,2007,43(10):4123−4135. doi: 10.1016/j.eurpolymj.2007.07.004
[26] 汪凯. 球磨法制备石墨烯——甲壳素纳米纤维杂化材料及其应用[D]. 青岛:青岛科技大学, 2018. [WANG K. The ball-milling preparation, functionalization of graphene/chitin nanofibers hybrids and their application[J]. Qingdao:Qingdao University of Science &Technology, 2018.] WANG K. The ball-milling preparation, functionalization of graphene/chitin nanofibers hybrids and their application[J]. Qingdao: Qingdao University of Science &Technology, 2018.
[27] NGASOTTER S, SAMPATH L, XAVIER K A M. Nanochitin:An update review on advances in preparation methods and food applications[J]. Carbohydr Polym,2022,291:119627. doi: 10.1016/j.carbpol.2022.119627
[28] SHAMSHINA J L, BERTON P, ROGERS R D. Advances in functional chitin materials:A review[J]. ACS Sustainable Chemistry & Engineering,2019,7(7):6444−6457.
[29] 孙绪兵, 杜京城, 由耀辉. 纳米甲壳素的制备、改性及应用研究进展[J]. 高分子通报,2016(8):71−80. [SUN X B, DU J C, YOU Y H. Research progress in the preparation, modification, and application of nanochitin[J]. Polymer Bulletin,2016(8):71−80.] SUN X B, DU J C, YOU Y H. Research progress in the preparation, modification, and application of nanochitin[J]. Polymer Bulletin, 2016(8): 71−80.
[30] KISHIMOTO M, IZAWA H, SAIMOTO H, et al. Dyeing of chitin nanofibers with reactive dyes and preparation of their sheets and nanofiber/resin composites[J]. Cellulose,2021,29(5):2829−2837.
[31] IFUKU S. Chitin and chitosan nanofibers:Preparation and chemical modifications[J]. Molecules,2014,19(11):18367−18380. doi: 10.3390/molecules191118367
[32] ZHANG X, ROLANDI M. Engineering strategies for chitin nanofibers[J]. J Mater Chem B,2017,5(14):2547−2559. doi: 10.1039/C6TB03324E
[33] ZHANG Y, JIANG J, LIU L, et al. Preparation, assessment, and comparison of α-chitin nano-fiber films with different surface charges[J]. Nanoscale Research Letters,2015,10(1):226. doi: 10.1186/s11671-015-0926-z
[34] ZOU H, LIN B, XU C, et al. Preparation and characterization of individual chitin nanofibers with high stability from chitin gels by low-intensity ultrasonication for antibacterial finishing[J]. Cellulose,2017,25(2):999−1010.
[35] LARBI F, GARCíA A, DEL VALLE L J, et al. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin:From energy production to material cytotoxicity and Pickering emulsion properties[J]. Carbohydrate Polymers,2018,196:385−397. doi: 10.1016/j.carbpol.2018.04.094
[36] ZHONG C, COOPER A, KAPETANOVIC A, et al. A facile bottom-up route to self-assembled biogenic chitin nanofibers[J]. Soft Matter,2010,6(21):5298. doi: 10.1039/c0sm00450b
[37] MALLIK A K, SAKIB M N, SHAHARUZZAMAN M, et al. Chitin nanomaterials:Preparation and surface modifications [M]. Holland: Elsevier Science RM Elsevier eBooks, 2020:165−194.
[38] IFUKU S, IKUTA A, HOSOMI T, et al. Preparation of polysilsesquioxane-urethaneacrylate copolymer film reinforced with chitin nanofibers[J]. Carbohydr Polym,2012,89(3):865−869. doi: 10.1016/j.carbpol.2012.04.022
[39] CHEN C, LI D, YANO H, et al. Bioinspired hydrogels:Quinone crosslinking reaction for chitin nanofibers with enhanced mechanical strength via surface deacetylation[J]. Carbohydr Polym,2019,207:411−417. doi: 10.1016/j.carbpol.2018.12.007
[40] GAO K, GUO Y, NIU Q, et al. Effects of chitin nanofibers on the microstructure and properties of cellulose nanofibers/chitin nanofibers composite aerogels[J]. Cellulose,2018,25(8):4591−4602. doi: 10.1007/s10570-018-1899-8
[41] ABDELRAHMAN R M, ABDEL-MOHSEN A M, ZBONCAK M, et al. Hyaluronan biofilms reinforced with partially deacetylated chitin nanowhiskers:Extraction, fabrication, in-vitro and antibacterial properties of advanced nanocomposites[J]. Carbohydrate Polymers,2020,235:115951. doi: 10.1016/j.carbpol.2020.115951
[42] HSUEH C Y, TSAI M L, LIU T. Enhancing saltiness perception using chitin nanofibers when curing tilapia fillets[J]. LWT,2017,86:93−98. doi: 10.1016/j.lwt.2017.07.057
[43] LU Y, SUN Q, SHE X, et al. Fabrication and characterisation of α-chitin nanofibers and highly transparent chitin films by pulsed ultrasonication[J]. Carbohydrate Polymers,2013,98(2):1497−1504. doi: 10.1016/j.carbpol.2013.07.038
[44] IFUKU. S, NOGI. M, ABE. K, et al. Preparation of chitin nanofibers with a uniform width as r-Chitin from crab shells[J]. Biomacromolecules,2009,10(6):1584−1588. doi: 10.1021/bm900163d
[45] AKLOG Y F, NAGAE T, IZAWA H, et al. Preparation of chitin nanofibers by surface esterification of chitin with maleic anhydride and mechanical treatment[J]. Carbohydr Polym,2016,153:55−59. doi: 10.1016/j.carbpol.2016.07.060
[46] SIAHKAMARI M, JAMALI A, SABZEVARI A, et al. Removal of Lead(II) ions from aqueous solutions using biocompatible polymeric nano-adsorbents:A comparative study[J]. Carbohydrate Polymers,2017,157:1180−1189. doi: 10.1016/j.carbpol.2016.10.085
[47] FAZLI WAN NAWAWI W M, LEE K Y, KONTTURI E, et al. Chitin nanopaper from mushroom extract:Natural composite of nanofibers and glucan from a single biobased source[J]. ACS Sustainable Chemistry & Engineering,2019,7(7):6492−6496.
[48] SALABERRIA A M, FERNANDES S C M, DIAZ R H, et al. Processing of α-chitin nanofibers by dynamic high pressure homogenization:Characterization and antifungal activity against A. niger[J]. Carbohydrate Polymers,2015,116:286−291. doi: 10.1016/j.carbpol.2014.04.047
[49] MUSHI N E, NISHINO T, BERGLUND L A, et al. Strong and tough chitin film from α-chitin nanofibers prepared by high pressure homogenization and chitosan addition[J]. ACS Sustainable Chemistry & Engineering,2018,7(1):1692−1697.
[50] WU C, LI Y, SUN J, et al. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging[J]. Food Hydrocolloids,2020,98:105245. doi: 10.1016/j.foodhyd.2019.105245
[51] WU C, SUN J, CHEN M, et al. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring[J]. Food Hydrocolloids,2019,95:308−317. doi: 10.1016/j.foodhyd.2019.04.047
[52] YE W, YOKOTA S, FAN Y, et al. A combination of aqueous counter collision and TEMPO-mediated oxidation for doubled carboxyl contents of α-chitin nanofibers[J]. Cellulose,2021,28(4):2167−2181. doi: 10.1007/s10570-021-03676-2
[53] YE W, HU Y, MA H, et al. Comparison of cast films and hydrogels based on chitin nanofibers prepared using TEMPO/NaBr/NaClO and TEMPO/NaClO/NaClO2 systems[J]. Carbohydrate Polymers,2020,237:116125. doi: 10.1016/j.carbpol.2020.116125
[54] XU Y, LIANG K, ULLAH W, et al. Chitin nanocrystal enhanced wet adhesion performance of mussel-inspired citrate-based soft-tissue adhesive[J]. Carbohydrate Polymers,2018,190:324−330. doi: 10.1016/j.carbpol.2018.03.005
[55] QIN Y, ZHANG S, YU J, et al. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films[J]. Carbohydr Polym,2016,147:372−378. doi: 10.1016/j.carbpol.2016.03.095
[56] OUN A A, RHIM J W. Effect of isolation methods of chitin nanocrystals on the properties of chitin-silver hybrid nanoparticles[J]. Carbohydr Polym,2018,197:349−358. doi: 10.1016/j.carbpol.2018.06.033
[57] DJALAL T, HAZWAN M H, MOHAMAD K M H. Recent progress in cellulose nanocrystals:Sources and production[J]. Nanoscale,2017,9(5):1749−2096. doi: 10.1039/C7NR90020A
[58] LIU L, SETA F T, AN X, et al. Facile isolation of colloidal stable chitin nano-crystals from Metapenaeus ensis shell via solid maleic acid hydrolysis and their application for synthesis of silver nanoparticles[J]. Cellulose,2020,27(17):9853−9875. doi: 10.1007/s10570-020-03499-7
[59] MOTA-MORALES J D, SáNCHEZ-LEIJA R J, CARRANZA A, et al. Free-radical polymerizations of and in deep eutectic solvents:Green synthesis of functional materials[J]. Progress in Polymer Science,2018,78:139−153. doi: 10.1016/j.progpolymsci.2017.09.005
[60] HONG S, YUAN Y, YANG Q, et al. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell[J]. Carbohydr Polym,2018,201:211−217. doi: 10.1016/j.carbpol.2018.08.059
[61] MUSHI N E, UTSEL S, BERGLUND L A. Nanostructured biocomposite films of high toughness based on native chitin nanofibers and chitosan[J]. Front Chem,2014,2:99.
[62] YUAN Y, HONG S, LIAN H, et al. Comparison of acidic deep eutectic solvents in production of chitin nanocrystals[J]. Carbohydr Polym,2020,236:116095. doi: 10.1016/j.carbpol.2020.116095
[63] NAGHDI T, GOLMOHAMMADI H, YOUSEFI H, et al. Chitin nanofiber paper toward optical (bio)sensing applications[J]. ACS Appl Mater Interfaces,2020,12(13):15538−15552. doi: 10.1021/acsami.9b23487
[64] TOPUZ F, UYAR T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications[J]. Food Research International,2020,130:108927. doi: 10.1016/j.foodres.2019.108927
[65] SHAMSHINA J L, ZAVGORODNYA O, CHOUDHARY H, et al. In search of stronger/cheaper chitin nanofibers through electrospinning of chitin-cellulose composites using an ionic liquid platform[J]. ACS Sustainable Chemistry & Engineering,2018,6(11):14713−14722.
[66] KADOKAWA J I, IDENOUE S, YAMAMOTO K. Fabricating chitin paper from self-assembled nanochitins[J]. ACS Sustainable Chemistry & Engineering,2020,8(22):8402−8408.
[67] LIAO J, ZHOU Y, HOU B, et al. Nano-chitin:Preparation strategies and food biopolymer film reinforcement and applications[J]. Carbohydrate Polymers,2023,305:120553. doi: 10.1016/j.carbpol.2023.120553
[68] WU C, JIANG H, ZHAO J, et al. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness[J]. Food Hydrocolloids,2022,133:107998. doi: 10.1016/j.foodhyd.2022.107998
[69] JIANG H, SUN J, LI Y, et al. Preparation and characterization of citric acid crosslinked konjac glucomannan/surface deacetylated chitin nanofibers bionanocomposite film[J]. Int J Biol Macromol,2020,164:2612−2621. doi: 10.1016/j.ijbiomac.2020.08.138
[70] DUAN M, YU S, SUN J, et al. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging[J]. International Journal of Biological Macromolecules,2021,187:332−340. doi: 10.1016/j.ijbiomac.2021.07.140
[71] SALABERRIA A M, DIAZ R H, ANDRES M A, et al. The antifungal activity of functionalized chitin nanocrystals in poly (lactid acid) films[J]. Materials (Basel),2017,10(5):546. doi: 10.3390/ma10050546
[72] XIE C, WANG F, HE Z, et al. Development and characterization of active packaging based on chitosan/chitin nanofibers incorporated with scallion flower extract and its preservation in fresh-cut bananas[J]. International Journal of Biological Macromolecules,2023,242:125045. doi: 10.1016/j.ijbiomac.2023.125045
[73] ZHANG X, WANG D, LIU S, et al. Bacterial cellulose nanofibril-based pickering emulsions:Recent trends and applications in the food industry[J]. Foods,2022,11(24):4064. doi: 10.3390/foods11244064
[74] SUN G, ZHAO Q, LIU S, et al. Complex of raw chitin nanofibers and zein colloid particles as stabilizer for producing stable pickering emulsions[J]. Food Hydrocolloids,2019,97:105178. doi: 10.1016/j.foodhyd.2019.105178
[75] WANG Y, YANG F, YANG J, et al. Synergistic stabilization of oil in water emulsion with chitin particles and tannic acid[J]. Carbohydr Polym,2021,254:117292. doi: 10.1016/j.carbpol.2020.117292
[76] ZHONG W, LI D, LI L, et al. pH-Responsive Pickering emulsion containing citrus essential oil stabilized by zwitterionically charged chitin nanofibers:Physicochemical properties and antimicrobial activity[J]. Food Chemistry,2024,433:137388. doi: 10.1016/j.foodchem.2023.137388
[77] DHANASEKARAN S, RAMESHTHANGAM P, VENKATESAN S, et al. In vitro and in silico studies of chitin and chitosan based nanocarriers for curcumin and insulin delivery[J]. Journal of Polymers and the Environment,2018,26(10):4095−4113. doi: 10.1007/s10924-018-1282-8
[78] PETROVA V A, ELOKHOVSKIY V Y, RAIK S V, et al. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile[J]. Biomolecules,2019,9(7):291. doi: 10.3390/biom9070291
[79] JIA X, MA P, TAYLOR K S Y, et al. Development of stable pickering emulsions with TEMPO-oxidized chitin nanocrystals for encapsulation of quercetin[J]. Foods,2023,12(2):367. doi: 10.3390/foods12020367
[80] TORLOPOV M A, VASENEVA I N, MIKHAYLOV V I, et al. Surface, rheopexy, digestive stability and toxicity of olive oil emulsions stabilized by chitin nanocrystals for vitamin D3 delivery[J]. Carbohydrate Polymers,2022,284:119162. doi: 10.1016/j.carbpol.2022.119162
[81] ZOU Y, ZHANG S, LIU Y, et al. In vitro digestion properties of different chitin nanofibrils stabilized lipid emulsions[J]. Food Hydrocolloids,2023,139:108512. doi: 10.1016/j.foodhyd.2023.108512
[82] ZHOU H, DAI T, LIU J, et al. Chitin nanocrystals reduce lipid digestion and β-carotene bioaccessibility:An in-vitro INFOGEST gastrointestinal study[J]. Food Hydrocolloids,2021,113:106494. doi: 10.1016/j.foodhyd.2020.106494
[83] BARAKI S Y, JIANG Y, LI X, et al. Stable sunflower oil oleogel from oil/water pickering emulsion with regenerated chitin[J]. LWT,2021,146:111483. doi: 10.1016/j.lwt.2021.111483
[84] ZHU Y, HUAN S, BAI L, et al. High internal phase oil-in-water pickering emulsions stabilized by chitin nanofibrils:3D structuring and solid foam [J]. ACS Applied Materials & Interfaces,2020,12(9):11240−11251.
[85] CUI S, LI M, ZHANG S, et al. Physicochemical properties of maize and sweet potato starches in the presence of cellulose nanocrystals[J]. Food Hydrocolloids,2018,77:220−227. doi: 10.1016/j.foodhyd.2017.09.037
[86] JIANG W J, TSAI M L, LIU T. Chitin nanofiber as a promising candidate for improved salty taste[J]. LWT,2017,75:65−71. doi: 10.1016/j.lwt.2016.08.050
[87] 陈悦, 陈超美, 刘则渊, 等. CiteSpace知识图谱的方法论功能[J]. 科学学研究,2015,33(2):242−253. [CHEN Y, CHEN C M, LIU Z Y, et al. The methodological function of CiteSpace knowledge graph[J]. Studies In Science of Science,2015,33(2):242−253.] doi: 10.3969/j.issn.1003-2053.2015.02.009 CHEN Y, CHEN C M, LIU Z Y, et al. The methodological function of CiteSpace knowledge graph[J]. Studies In Science of Science, 2015, 33(2): 242−253. doi: 10.3969/j.issn.1003-2053.2015.02.009
[88] REN M, YU X, MUJUMDAR A S, et al. Visualizing the knowledge domain of pulsed light technology in the food field:A scientometrics review[J]. Innovative Food Science & Emerging Technologies,2021,74:102823.





 下载:
下载:
 下载:
下载:
