Influence of Key Processing Technology on the Quality of Freshly-squeezed Lettuce Juice
-
摘要: 本研究以水培绿萝莎生菜为原料,研究漂烫(95 ℃/2 min)、分离(6000×g/10 min)、杀菌(600 MPa,25 ℃/2 min)三个关键生产工艺对鲜榨生菜汁微生物、色泽参数、理化指标、酶活性及抗氧化能力的影响。结果表明,经杀菌工艺后鲜榨生菜汁总好氧菌小于2 lgCFU/mL,霉菌和酵母菌小于1.3 lgCFU/mL,均在国标(GB 7101-2015)限量范围内。分离工艺显著影响生菜汁色泽和叶绿素含量,鲜榨清汁的L*、a*、b*和C*值均显著升高(P<0.05),清汁更加明亮、绿色变浅、黄色加深、饱和度升高,总叶绿素含量下降了56.44%。漂烫后鲜榨生菜汁a*值显著降低(P<0.05),绿色更加明亮,漂烫浊汁的多酚氧化酶、过氧化物酶和过氧化氢酶失活率分别为83.76%、84.87%和90.80%。漂烫和杀菌均影响鲜榨生菜汁的活性成分和抗氧化能力,可溶性糖含量保留率分别为91.24%和88.3%,可溶性蛋白的保留率为64.22%和60.60%,总酚含量分别损失了53.31%和56.28%,抗氧化能力降低了82.61%和73.91%。本研究可为鲜榨蔬菜汁加工提供技术参考。Abstract: In order to realize the effects of blanching (95 ℃/2 min), separation (6000×g/10 min) and sterilization (600 MPa, 25 ℃/2 min) on the processing quality of freshly-squeezed lettuce juice, hydroponic green rosa lettuce were chosen as the test material. Changes of microorganisms, color, physical and chemical parameters, enzyme activities and antioxidant capacity were evaluated. The results suggested that the total aerobic bacteria of freshly-squeezed lettuce juice was less than 2 lgCFU/mL and the mold and yeast were less than 1.3 lgCFU/mL after sterilization process, all of which were within the national standard limit (GB 7101-2015). Separation process significantly affected the color and chlorophyll content of lettuce juice. The L*, a*, b* and C* values of fresh clear lettuce juice were significantly increased (P<0.05), and the clear juice was brighter, lighter in green, deeper in yellow and higher in saturation, and the total chlorophyl content decreased by 56.44%. Blanching process significantly decreased the a* value (P<0.05), and the green color became brighter. The inactivation rates of polyphenoloxidase, peroxidase and catalase were 83.76%, 84.87% and 90.80% after blanching, respectively. Blanching and sterilization both affected the active components and antioxidant capacity of freshly-squeezed lettuce juice, the soluble sugar content retention rate was 91.24% and 88.3%, the soluble protein retention rate was 64.22% and 60.60%, the total phenolic content was decreased by 53.31% and 56.28%, the antioxidant capacity decreased by 82.61% and 73.91%, respectively. This study could provide technical reference for processing of freshly-squeezed vegetable juice.
-
叶用莴苣(Lactuca sativa L.)俗称生菜,是最常见的生食叶菜,维生素和膳食纤维含量丰富,具有调节酸碱平衡、提高免疫力、润肠通便等功能[1]。但因其组织脆嫩、含水量高,采后极易失水萎蔫、黄化、腐烂变质,导致货架期短[2]。通过机械物理等技术手段开发生菜加工产品,不仅可以有效解决生菜产量过剩、采后腐烂等问题,还能丰富蔬菜加工制品种类[3]。鲜榨果蔬汁有“液体果蔬”之称,可作为即食新鲜果蔬的替代品。现行农业行业标准《非浓缩还原果蔬汁加工技术规程》(NY/T 3909-2021)[4]中明确,以水果、蔬菜为原料,通过机械方法直接制成的可发酵但未发酵的汁液制品为非浓缩还原(Not from concentrate,NFC)果蔬汁,而采用超高压等非热杀菌或巴氏杀菌的NFC果蔬汁产品也可标注为“鲜榨果蔬汁”。当前市场以鲜榨水果汁为主,相比之下,鲜榨蔬菜汁具有低糖、低热量、高膳食纤维等独特优势,更符合未来消费者对天然风味和营养健康的需求[5]。
不同于果汁加工工艺,蔬菜膳食纤维含量高、出汁率低[6],且鲜榨加工过程中极易氧化褪绿,导致色泽等品质下降[7]。漂烫处理一方面软化蔬菜组织提高出汁率,另一方面可以钝化多酚氧化酶(Polyphenoloxidase,PPO)和过氧化物酶(Peroxidase,POD)等活性[8],抑制色素氧化,使色素更容易溶出,降低营养成分的损失[9−14]。蔬菜汁破碎制汁后含有果胶、蛋白质、膳食纤维等悬浮物质,相互作用后形成不溶性的絮状物质而产生沉淀,影响蔬菜汁的感官品质、贮藏性及商品性等。分离澄清技术可去除蔬菜汁中的果胶和悬浮物等,使得产品清透并且保持良好的感官品质。杀菌是鲜榨生菜汁加工的必要和关键环节,直接影响蔬菜汁的品质和货架期[15]。区别于传统温度较高或时间较长的热杀菌造成鲜榨汁色泽、风味、活性成分等的损失,超高压(Ultra-high pressure,UHP)技术将果蔬汁密封包装在密闭容器中,在100~1000 MPa的静压力下使果蔬制品中的细胞破碎、蛋白质变性、淀粉糊化,并达到杀菌效果[16],可最大程度保留产品原有的感官品质和营养价值[17]。黄瓜汁饮料在500 MPa/2 min的高压处理后叶绿素含量与对照组无显著差异,而85 ℃/15 s的热处理后叶绿素含量显著下降[18]。
然而,以绿色叶菜为来源的鲜榨蔬菜汁尤其是鲜榨生菜汁市场缺乏,关键生产工艺对其制汁特性、品质变化影响不明。因此,本实验以绿萝莎生菜为原料,探究漂烫、分离、杀菌三个关键生产工艺对鲜榨生菜汁色泽参数、理化指标、叶绿素含量、酶活性及抗氧化能力的影响,阐明不同加工工艺对鲜榨生菜汁感官及品质的影响,为鲜榨生菜汁规模化加工的质量控制和品质提升提供理论依据。
1. 材料与方法
1.1 材料与仪器
绿萝莎水培生菜 采于北京创世大业科技有限公司工业园区,选取绿色新鲜、生长周期(25±1)d、重量为(120±5)g的绿萝莎生菜,当天采摘2 h内运回实验室,现采现用;平板计数琼脂、马铃薯葡萄糖琼脂 北京奥博星生物技术有限公司;无水乙醇、丙酮、浓硫酸、磷酸 国药集团化学试剂有限公司;牛血清白蛋白标准品、考马斯亮蓝G-250 北京索莱宝科技有限公司;植物可溶性糖、总酚含量试剂盒、过氧化氢酶试剂盒、多酚氧化酶试剂盒、超氧阴离子清除能力试剂盒、总抗氧化能力试剂盒、羟基自由基清除能力试剂盒 苏州科铭生物技术有限公司。
L12-P126型高速破壁调理机 北京合千润科贸有限公司;JML型胶体磨 温州市弘安机械有限公司;PAL-BX/ACID 5型糖酸度计 日本爱拓公司;DigiEye型电子眼 英国Verivide公司;Sigma3k15台式高速冷冻分离机 上海斯高勒生物科技有限公司;FiveEasy Plus pH计 梅特勒-托利多仪器(上海)有限公司。
1.2 实验方法
1.2.1 生菜汁的制备
鲜榨浊汁:选取无机械损伤的新鲜水培生菜,去根,清洗,沥干水分,切段,打浆,过胶体磨,静置1 h,2层100目过筛。
鲜榨清汁:将鲜榨生菜浊汁于6000×g下4 ℃分离10 min,取上清,即得。漂烫浊汁:选取无机械损伤的新鲜水培生菜,去根,清洗,沥干水分,切段,沸水中漂烫2 min,过冷水,沥干水分,打浆,过胶体磨,静置1 h,2层100目过筛,即得。漂烫清汁:将漂烫生菜浊汁于6000×g下4 ℃分离10 min,取上清,即得。杀菌浊汁:将漂烫生菜浊汁罐装于棕色瓶中,25 ℃下600 MPa保压120 s,即得。杀菌清汁:将漂烫生菜清汁罐装于棕色瓶中,25 ℃下600 MPa保压120 s,即得。
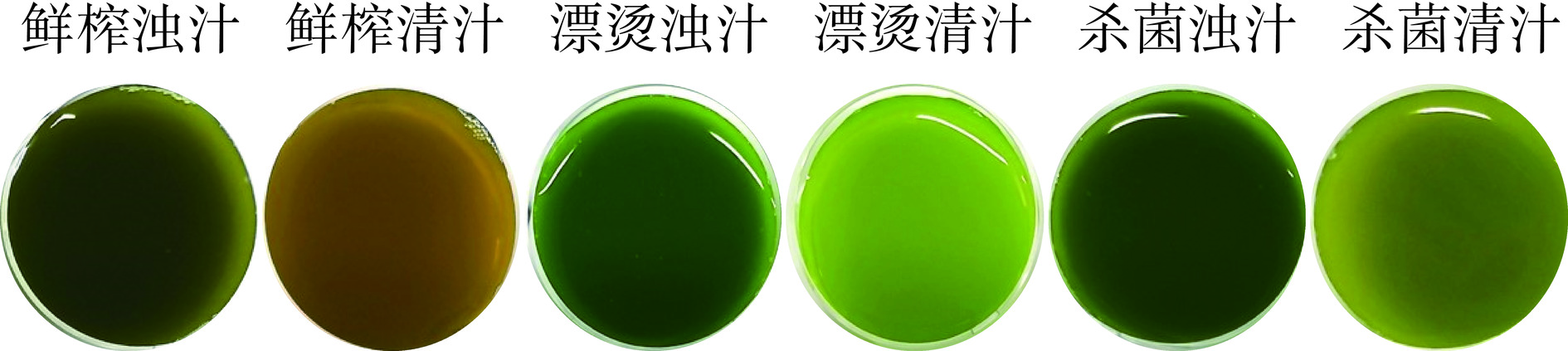
将加工过程中得到的六种生菜汁样品如图1所示,将其罐装于棕色玻璃瓶中,一部分置于4 ℃冰箱,鲜样待测(不超过3 d)。另一部分生菜汁样品液氮速冻,置于−80 ℃待测。
1.2.2 微生物测定
根据(GB 4789.2-2016和GB 4789.15-2016)[19−20]测定鲜榨生菜汁样品中的总好氧菌(Total aerobic bacteria,TAB)及酵母菌和霉菌(Yeasts and molds,Y&M),分别吸取1 mL样品,平皿计数琼脂和马铃薯葡萄糖琼脂倾注平皿。总好氧菌于(36±1)℃培养箱中培养(48±2)h。霉菌和酵母菌于(28±1)℃培养箱中培养(5±2)d。结果以每毫升生菜汁菌落形成单位数的十进制对数表示,为lgCFU/mL。
1.2.3 色泽测定
采用电子眼测定生菜汁的色度值,吸取5 mL生菜汁样品于35 mm的培养皿中,电子眼拍照,分析生菜汁样品的L*(明/暗)、a*(红/绿)、b*(黄/蓝)、C*(Chroma,饱和度)和h(Hue angle,色调)值。
1.2.4 可溶性固形物(Total soluble solids,TSS)、酸度(Acid)、pH测定
TSS、Acid通过手持式糖酸度仪测量,pH用pH计检测。
1.2.5 叶绿素含量测定
参照Zhao等[21]和El-nakhel等[22]方法检测生菜汁样品的叶绿素a、叶绿素b和总叶绿素的含量。将500 mL的生菜汁在10 mL的丙酮(90%)中研磨,然后在3000×g下离心10 min。收集上清液并再次加入丙酮(90%),直到达到25 mL的体积。用分光光度计在662、645 nm的两波长度下分别测量叶绿素a和b的吸光度。总叶绿素被计算为叶绿素a和b的总和。叶绿素a和b的浓度用以下转换公式计算。
1.2.6 可溶性糖含量测定
依据植物可溶性糖含量试剂盒说明书测定样品的可溶性糖含量。
1.2.7 可溶性蛋白含量测定
牛血清白蛋白为标准蛋白,绘制标准曲线为y=0.0037x+0.0002,R2=0.9996。采用考马斯亮蓝法测定[23],计算可溶性蛋白含量。
式中:m′—标曲中查得的蛋白含量,µg;V—样品提取液体积,mL;Vs—测定时所取样品提取液体积,mL;m—样品质量,g。
1.2.8 总酚含量测定
依据总酚试剂盒说明书测定生菜汁样品的总酚含量。
1.2.9 POD、PPO、CAT活性测定
将生菜汁样品液氮研磨并准确称取0.1 g,加入粗酶提取液(含1 mmoL PEG、4% PVPP和1% Triton X-100)0.5 mL,在4 ℃下分离10 min,得上清液,依据试剂盒说明书测定POD、PPO、CAT活性。
1.2.10 超氧阴离子自由基清除率、羟基自由基清除率和总抗氧化能力测定
依据试剂盒说明书测定超氧阴离子清除能力、羟基自由基清除能力、总抗氧化能力(DPPH法)。
1.3 数据处理
所有实验进行3~6次重复,数值为平均值±标准偏差,采用OriginPro 9.0软件进行数据处理与绘图。采用SPSS 24.0软件检验不同样品间差异显著性,小写字母不同表示在P<0.05水平上有显著差异。
2. 结果与分析
2.1 关键生产工艺对生菜汁微生物的影响
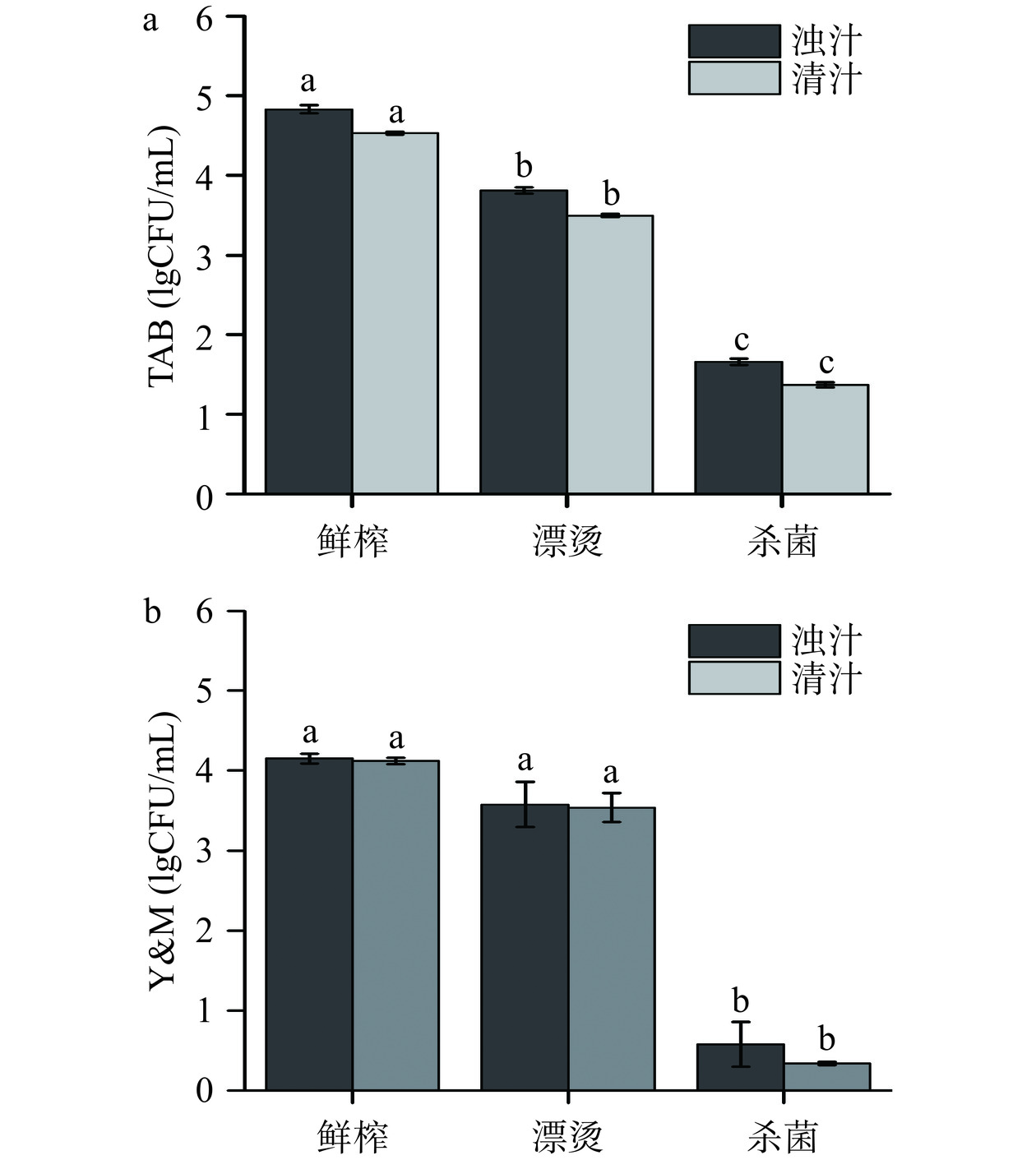
微生物指标是评价果蔬汁安全性的基础指标,食品安全国家标准《饮料》(GB 7101-2015)中微生物限量为:菌落总数≤2 lgCFU/mL、霉菌和酵母菌≤1.30 lgCFU/mL[24]。如图2所示,未处理组鲜榨生菜汁的初始TAB和Y&M分别为4.83±0.05、4.15±0.06 lgCFU/mL,均在国标安全限量以上,存在较高的微生物安全风险。分离工艺对生菜汁的TAB和Y&M无显著影响(P>0.05)。漂烫工艺显著降低了生菜汁的TAB(P<0.05),而对Y&M无显著影响(P>0.05),漂烫对微生物的影响源于高温导致蛋白质变性、代谢酶失活以及DNA损伤而杀灭微生物[25]。经超高压杀菌工艺后,生菜汁的TAB和Y&M显著降低(P<0.05),达到商业无菌状态,该结果归因于超高压力使微生物代谢酶和蛋白质的失活变性及对微生物细胞形态及结构完整性的破坏[5]。
2.2 关键生产工艺对生菜汁色泽的影响
色泽直接影响鲜榨生菜汁的商品性和消费者的购买接受度。如图1和表1所示,漂烫、分离和杀菌三种工艺均对生菜汁的L*、a*、b*、C*和h值等造成不同程度的影响。分离后的鲜榨清汁L*、a*、b*和C*值均显著升高(P<0.05),清汁更加明亮、绿色变浅、黄色加深和饱和度升高,这主要是由于清汁中的果肉含量少,叶绿素主要存在于果肉中,分离使得显示绿色的叶绿素发生沉降,清汁中的叶绿素含量减少,绿色变浅而黄色加深。漂烫后生菜汁的L*、b*、C*和h值均升高,漂烫使得生菜汁更加明亮、黄色加深、颜色更加饱和且色调更加均匀,但是漂烫后生菜汁的a*值显著下降(P<0.05)。杀菌后生菜汁的a*值升高,表明杀菌使得生菜汁的绿色变浅。漂烫和杀菌使生菜汁的绿色更加浓郁,这可能是由于叶绿体中不同成分在高温高压下的分布变动,或者高温使得叶绿素蛋白复合体中的蛋白变性导致叶绿素与蛋白质分离生成游离叶绿素[26]。
表 1 鲜榨生菜汁色泽参数变化Table 1. Changes of color parameters in freshly-squeezed lettuce juice生菜汁样品 L* a* b* C* h 鲜榨浊汁 27.82±0.45e -4.55±0.15b 14.35±0.28e 15.05±0.30e 107.58±0.41c 鲜榨清汁 41.61±0.41c 2.02±0.14a 30.42±0.55c 30.48±0.56c 86.21±0.20f 漂烫浊汁 37.14±0.18d -12.87±0.13f 17.78±0.23d 21.95±0.26d 125.92±0.12a 漂烫清汁 58.18±0.35a -9.32±0.13d 47.26±0.76a 48.17±0.75a 101.16±0.22d 杀菌浊汁 36.52±0.25d -11.47±0.10e 18.66±0.10d 21.90±0.13d 121.59±0.12b 杀菌清汁 51.10±1.11b -6.59±0.23c 40.24±1.68b 40.78±1.63b 99.32±0.64e 注:同列数据上标字母不同表示鲜榨生菜汁色度值存在显著性差异(P<0.05);n=6。 2.3 关键生产工艺对生菜汁理化指标的影响
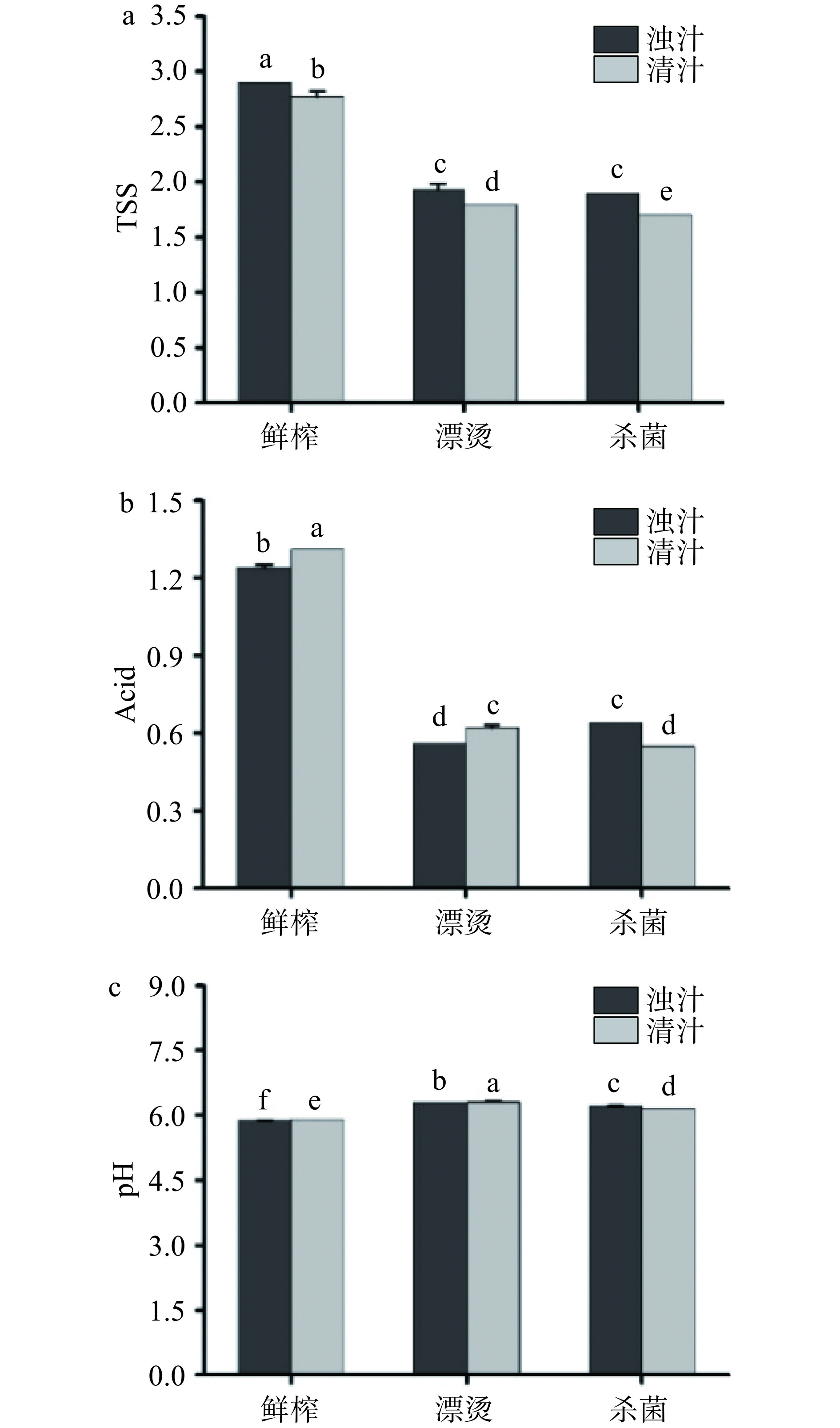
TSS由游离或组合形式的还原糖和非还原糖组成,酸度表示酚酸和有机酸等的含量,糖酸比直接影响生菜汁的口感和风味[27]。生菜汁中叶绿素降解包括酶促褐变和非酶促反应,其中非酶促反应中光、热、氧和pH均会诱导叶绿素降解[28]。如图3所示,三种工艺均对生菜汁的TSS和Acid影响较为显著(P<0.05),而对pH的影响变化不大。图3(a)显示分离后鲜榨清汁的TSS变化不大,漂烫和杀菌后生菜汁的TSS显著下降(P<0.05),可能是由于漂烫和高压造成植物细胞壁和细胞膜破裂,导致细胞内溶物质释放[29−30]。图3(b)显示分离后鲜榨清汁的Acid变化较小,漂烫和杀菌后生菜汁的Acid显著下降(P<0.05),漂烫和杀菌显著影响生菜汁的Acid含量。图3(c)显示,三种关键工艺对生菜汁pH影响变化不明显,均在6左右,表明加工过程对生菜汁的pH影响较小,这与Helena等[31]在菲油果汁加工中的研究结果一致。
2.4 关键生产工艺对生菜汁叶绿素含量的影响
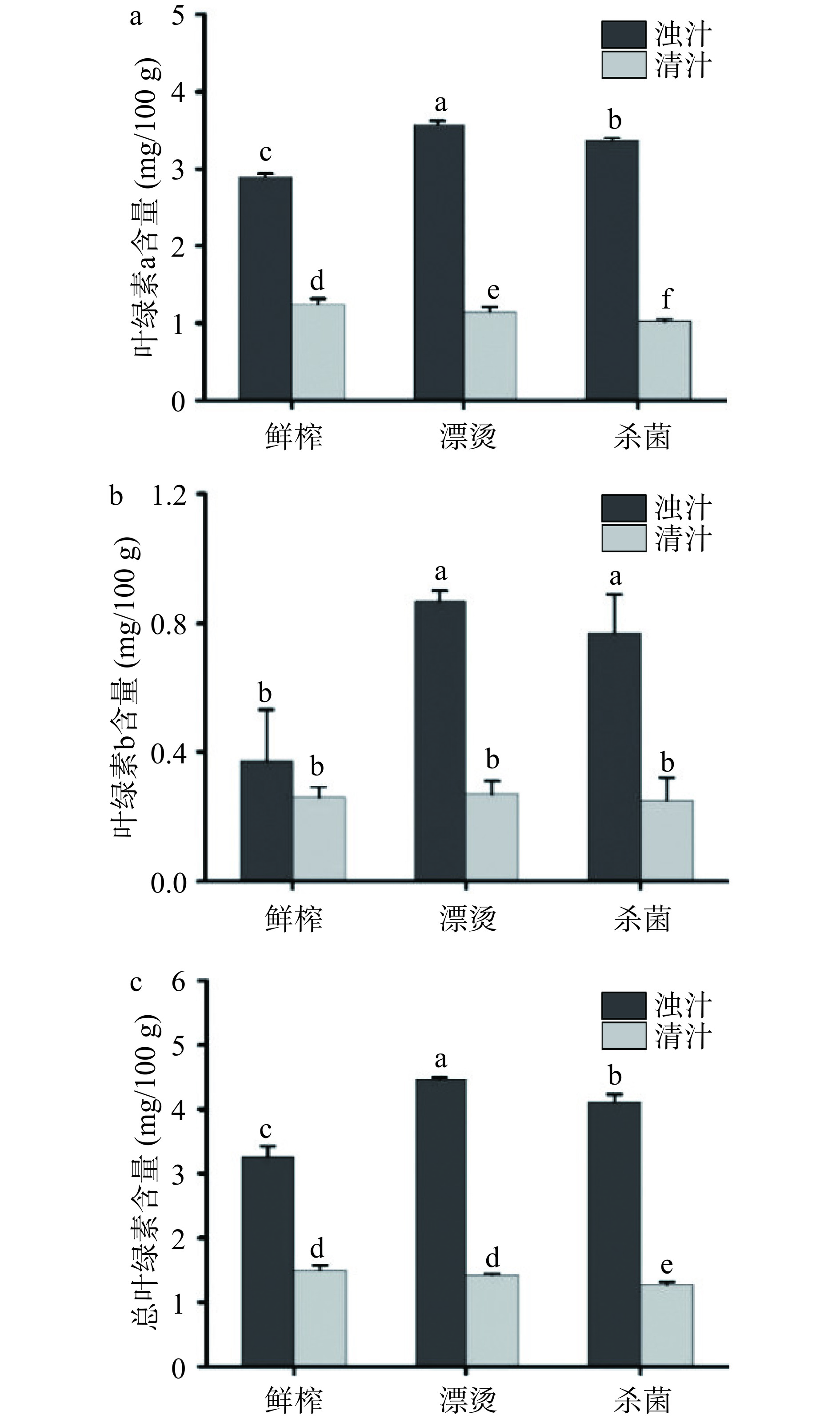
与其他果蔬汁相比,生菜汁色泽为绿色是该产品的一大亮点和特色,而叶绿素是影响鲜榨生菜汁颜色变化的主要指标之一[32]。如图4所示,分离后的清汁叶绿素a、叶绿素b和总叶绿素含量显著下降(P<0.05),其中鲜榨浊汁的总叶绿素含量从3.26±0.16 mg/100 g下降到1.42±0.07 mg/100 g,表明分离对生菜汁叶绿素含量影响显著,主要原因是分离使叶绿体沉降,导致叶绿素含量减少。Xu等[33]研究表明猕猴桃浊汁的叶绿素含量经分离工艺后降低了79.8%,与本研究结果一致。漂烫和杀菌后生菜浊汁的叶绿素a、叶绿素b和总叶绿素含量均显著升高(P<0.05),漂烫和杀菌后浊汁的总叶绿素含量分别为 4.46±0.04 mg/100 g和4.13±0.10 mg/100 g,分别升高了36.81%和26.69%,这可能是由于高温高压使得细胞结构、细胞器膜等破坏,游离叶绿素溶出增加[34]。Sánchez等[35]研究发现高压处理使西兰花叶绿素b含量增加了20%、菠菜叶绿素a含量增加了15%,与本研究结果一致。
2.5 关键生产工艺对生菜汁可溶性糖、可溶性蛋白和总酚含量的影响
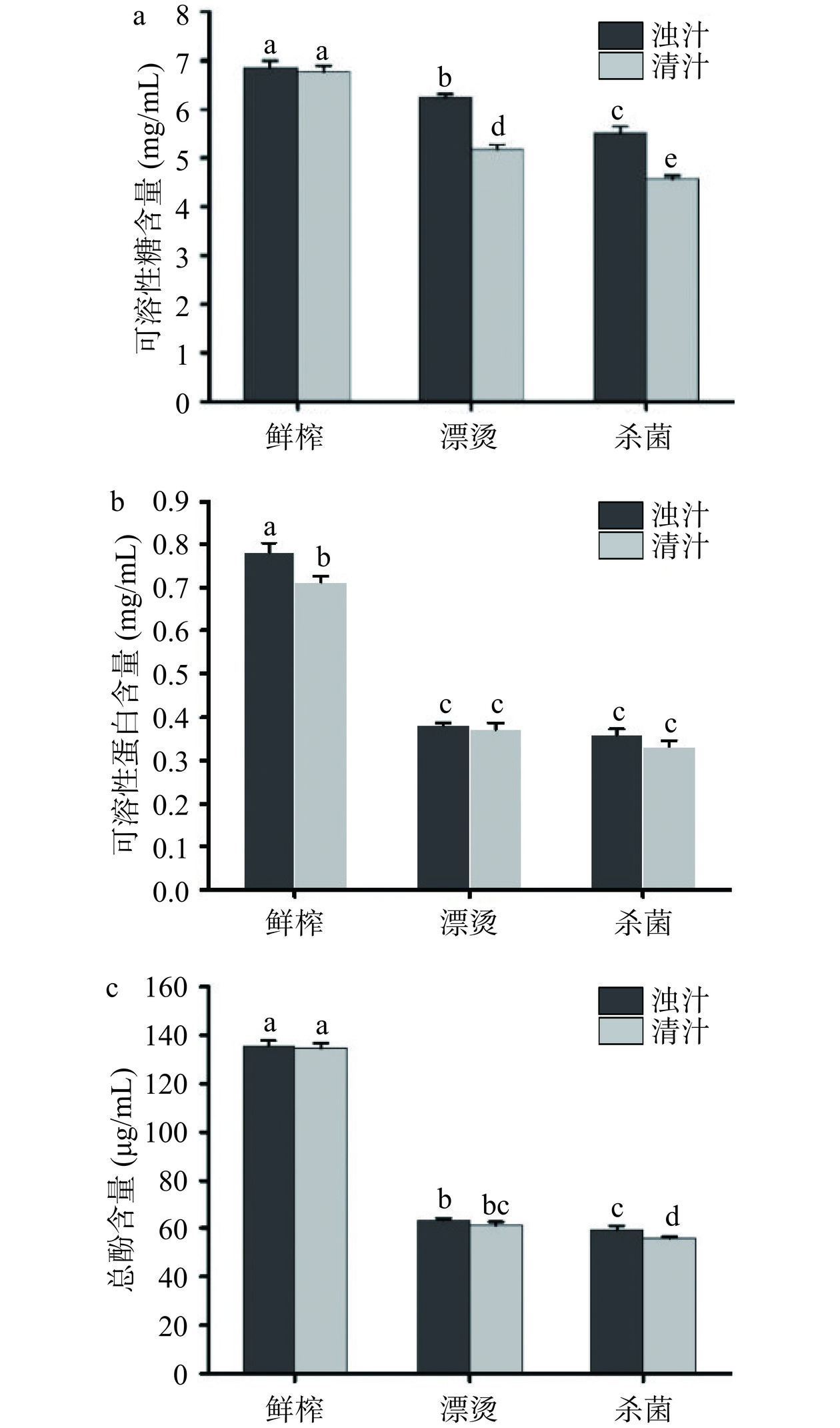
生菜汁可溶性糖、可溶性蛋白和总酚含量变化如图5所示。图5(a)显示,分离后鲜榨清汁的可溶性糖无显著变化(P>0.05),清汁可溶性糖的保留率达到98.8%,因此分离对鲜榨生菜汁可溶性糖含量影响不显著。漂烫和杀菌后生菜浊汁的可溶性糖含量显著降低(P<0.05),保留率分别为91.24%和88.3%,可能是漂烫使得可溶性糖溶于漂烫液中以及高温高压使得糖结构发生变化。图5(b)显示,与鲜榨浊汁相比,分离后鲜榨清汁的可溶性蛋白含量显著降低(P<0.05),同时漂烫和杀菌后生菜浊汁的可溶性蛋白含量也显著降低(P<0.05)。漂烫浊汁和杀菌浊汁的可溶性蛋白的保留率分别为64.22%和60.60%,表明漂烫和杀菌显著影响生菜汁的可溶性蛋白含量,可能与高温高压使得可溶性蛋白变性或溶出于漂烫液中有关[36]。
酚类化合物具有还原性、隔离活性氧、抗氧化和调节某些细胞酶活性等能力。图5(c)显示,分离对生菜汁总酚含量无显著影响(P>0.05),漂烫和杀菌后浊汁的总酚含量显著降低(P<0.05)。其中,漂烫使总酚含量从135.61±2.14 µg/mL降低到63.31±0.78 µg/mL,降低了53.31%,杀菌使总酚含量从135.61±2.14 µg/mL降低到59.28±1.71 µg/mL,降低了56.28%。先前研究表明,微波漂烫显著降低了芒果、苹果、香蕉和橙子的果皮总酚含量[37]。总酚含量的下降可能是漂烫和杀菌使通过醚、酯和碳-碳键与结构蛋白、纤维素和果胶等大分子结合酚类化合物的结合结构被破坏而释放、多酚类化合物降解转化,影响了酚类化合物的组成[38]。
2.6 关键生产工艺对生菜汁酶活性的影响
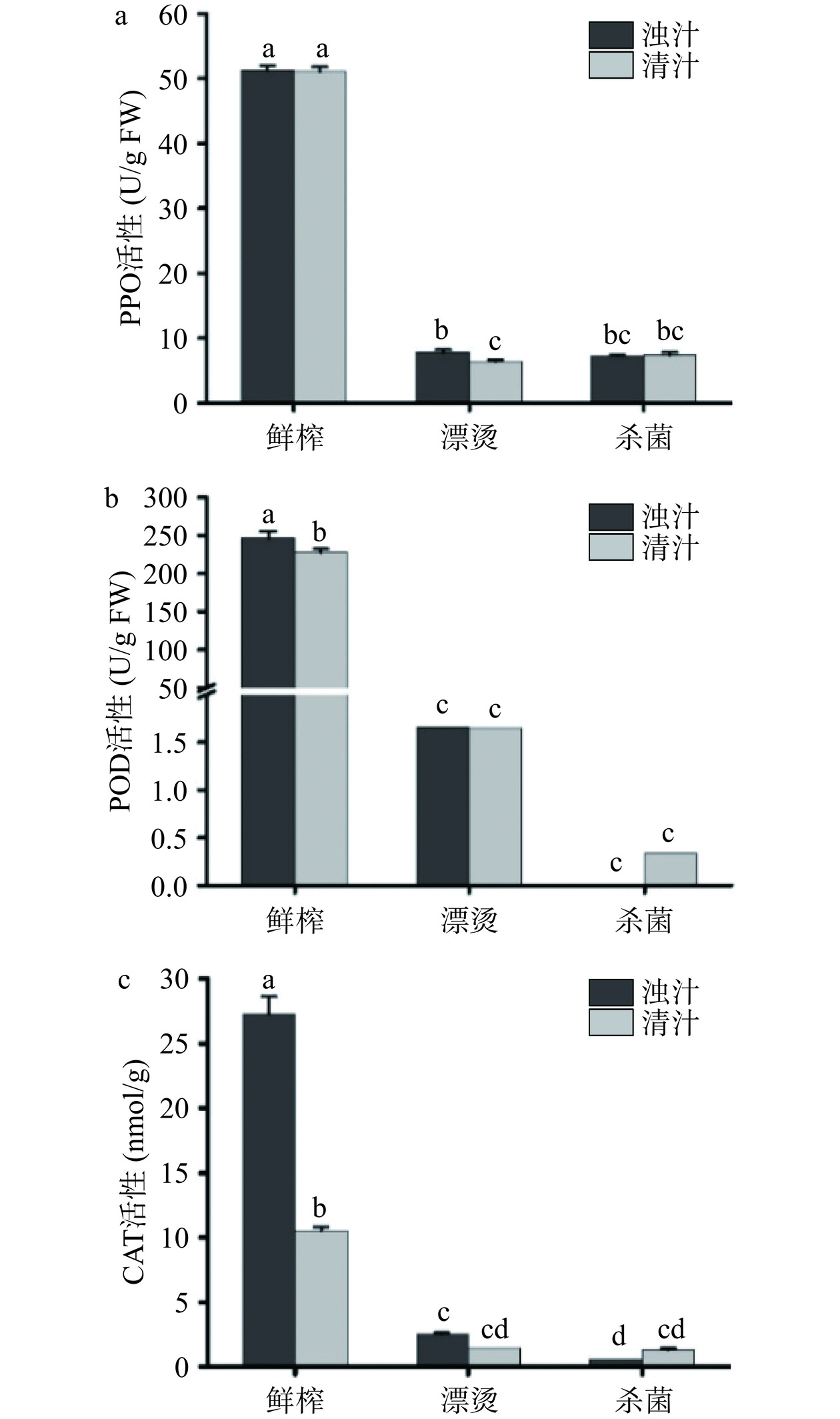
酶促褐变是造成生菜汁色泽变化的因素之一,主要由PPO和POD在氧气条件下将生菜汁中的酚类物质氧化为活性较强的醌类物质,并进一步发生褐变[39]。CAT广泛存在于植物体中,用于去除过氧化氢,有利于生菜汁的抗氧化性。关键生产工艺对生菜汁PPO、POD和CAT酶活性的影响如图6所示,分离后鲜榨清汁的PPO活性无显著变化(P>0.05),表明PPO主要存在于生菜汁溶液中,而分离后清汁的POD和CAT活性分别降低了8.16%和61.32%,表明POD和CAT主要存在于悬浮物质和果肉中,分离降低了二者活性。漂烫和杀菌后浊汁的PPO、POD和CAT三种酶活性显著降低(P<0.05),漂烫使PPO、POD和CAT的失活率分别达到83.76%、84.87%和90.80%,杀菌使PPO、POD和CAT的失活率分别为85.83%、99.10%和97.83%。漂烫和杀菌对生菜汁酶活性的作用显著,这是由于高温高压使酶结构改变、活性钝化。先前同样有研究报道漂烫工艺显著降低了甘蔗汁的PPO和POD活性[40]。
2.7 关键生产工艺对生菜汁抗氧化能力的影响
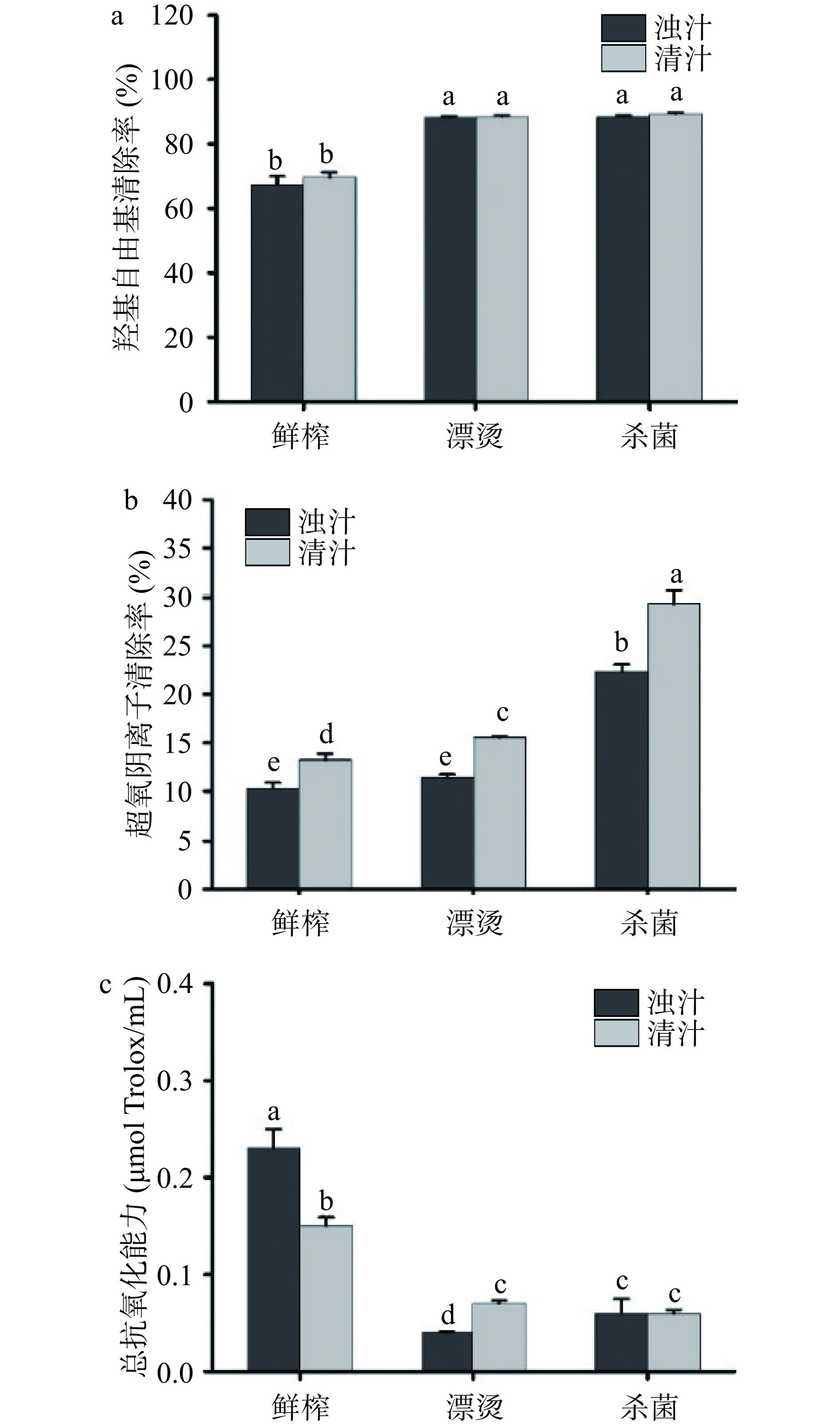
关键生产工艺对生菜汁抗氧化能力的影响如图7所示,分离对生菜汁的羟基自由基清除率无显著影响(P>0.05)。漂烫后浊汁的超氧阴离子清除率略有上升,而羟基自由基清除率显著升高(P<0.05),表明漂烫可以提高生菜汁的羟基自由基清除率。杀菌后浊汁的羟基自由基清除率和超氧阴离子清除率均显著上升(P<0.05)。鲜榨浊汁的抗氧化能力为0.23±0.02 µmol Trolox/mL,分离、漂烫和杀菌后生菜汁的抗氧化能力分别降低了34.78%、82.61%和73.91%,漂烫和杀菌均显著降低了生菜汁的抗氧化能力(P<0.05)。先前研究表明,超高压及高温短时处理均降低了菠萝果汁中的抗氧化能力[41],与本研究结果一致。生菜汁的抗氧化活性降低可能与酚类化合物的损失有关[42]。
3. 结论
本研究从微生物、色泽参数、理化指标、营养物质等方面探究了漂烫(95 ℃/2 min)、分离(6000×g/10 min)、杀菌(600 MPa,25 ℃/2 min)三个关键生产工艺对鲜榨生菜汁品质的影响。结果表明,分离和漂烫后的生菜汁仍存在微生物安全风险,而经杀菌工艺后鲜榨生菜汁总好氧菌小于2 lgCFU/mL,霉菌和酵母菌小于1.3 lgCFU/mL,达到商业无菌状态。因此,杀菌是鲜榨生菜汁加工的必要环节。分离工艺显著影响生菜汁的色泽参数,鲜榨清汁色泽更加明亮、绿色变浅、饱和度升高,总叶绿素含量下降了56.44%。漂烫工艺对鲜榨生菜汁PPO、POD和CAT的失活率分别达到83.76%、84.87%和90.80%,a*值显著降低,绿色更加明亮。因此,漂烫主要通过钝化生菜内源性酶活性,保持生菜汁鲜绿色泽。此外,漂烫和杀菌工艺均显著影响了生菜汁的活性成分和抗氧化能力,总酚含量分别损失了53.31%和56.28%,抗氧化能力分别降低了82.61%和73.91%。本研究可为鲜榨生菜汁规模化加工的质量控制和品质提升提供理论依据,研究结果也可推广应用到其他叶类蔬菜加工。
-
表 1 鲜榨生菜汁色泽参数变化
Table 1 Changes of color parameters in freshly-squeezed lettuce juice
生菜汁样品 L* a* b* C* h 鲜榨浊汁 27.82±0.45e -4.55±0.15b 14.35±0.28e 15.05±0.30e 107.58±0.41c 鲜榨清汁 41.61±0.41c 2.02±0.14a 30.42±0.55c 30.48±0.56c 86.21±0.20f 漂烫浊汁 37.14±0.18d -12.87±0.13f 17.78±0.23d 21.95±0.26d 125.92±0.12a 漂烫清汁 58.18±0.35a -9.32±0.13d 47.26±0.76a 48.17±0.75a 101.16±0.22d 杀菌浊汁 36.52±0.25d -11.47±0.10e 18.66±0.10d 21.90±0.13d 121.59±0.12b 杀菌清汁 51.10±1.11b -6.59±0.23c 40.24±1.68b 40.78±1.63b 99.32±0.64e 注:同列数据上标字母不同表示鲜榨生菜汁色度值存在显著性差异(P<0.05);n=6。 -
[1] SAWATDEE S, PROMMUAK C, JARUNGLUMLERT T, et al. Combined effects of cations in fertilizer solution on antioxidant content in red lettuce (Lactuca sativa L.)[J]. Journal of the Science of Food and Agriculture,2021,101(11):4632−4642. doi: 10.1002/jsfa.11106
[2] 刘才宇, 朱培蕾, 赵贵云, 等. 叶菜类蔬菜贮藏保鲜技术研究进展[J]. 安徽农业大学学报,2011,38(5):797−801. [LIU C Y, ZHU P L, ZHAO G Y, et al. Research progress on storage and freshness keeping technology of leaf vegetables[J]. Journal of Anhui Agricultural University,2011,38(5):797−801.] LIU C Y, ZHU P L, ZHAO G Y, et al. Research progress on storage and freshness keeping technology of leaf vegetables[J]. Journal of Anhui Agricultural University, 2011, 38(5): 797−801.
[3] 李芝萱, 钞春明, 张佳龙, 等. 不同温度对超高压处理鲜榨生菜汁贮藏稳定性的影响[J]. 食品科学,2023,44(1):78−87. [LI Z X, CHAO C M, ZHANG J L, et al. Effect of different storage temperatures on storage stability of fresh lettuce juice treated with ultra-high pressure[J]. Food Science,2023,44(1):78−87.] LI Z X, CHAO C M, ZHANG J L, et al. Effect of different storage temperatures on storage stability of fresh lettuce juice treated with ultra-high pressure[J]. Food Science, 2023, 44(1): 78−87.
[4] 中华人民共和国农业农村部. NY/T 3909-2021 非浓缩还原果蔬汁加工技术规程[S]. 北京:中国农业出版社, 2021. [Ministry of Agriculture and Rural Affairs of the People's Republic of China. NY/T 3909-2021 Technical specification for processing of non concentrated reduced fruit and vegetable juice[S]. Beijing:China Agricultural Publishing House, 2021.] Ministry of Agriculture and Rural Affairs of the People's Republic of China. NY/T 3909-2021 Technical specification for processing of non concentrated reduced fruit and vegetable juice[S]. Beijing: China Agricultural Publishing House, 2021.
[5] PLAZZOTTA S, MANZOCCO L. High-pressure homogenisation combined with blanching to turn lettuce waste into a physically stable juice[J]. Innovative Food ence & Emerging Technologies, 2018, 52:136−144.
[6] ALKORTA I, GARBISU C, LLAMA M J, et al. Industrial applications of pectic enzymes:A review[J]. Process Biochemistry,1998,33(1):21−28. doi: 10.1016/S0032-9592(97)00046-0
[7] YAN S L, YANG T B, LUO Y G. The mechanism of ethanol treatment on inhibiting lettuce enzymatic browning and microbial growth[J]. Food Science Technology,2015,63:383−390.
[8] SONG J, AN G, KIM C. Color, texture, nutrient contents, and sensory values of vegetable soybeans [Glycine max (L.) Merrill] as affected by blanching[J]. Food Chemistry,2003,83:69−74. doi: 10.1016/S0308-8146(03)00049-9
[9] TIJSKENS L M M, RODIS P S, HERTOG M L A T, et al. Activity of peroxidase during blanching of peaches, carrots and potatoes[J]. Journal of Food Engineering,1997,34:355−370. doi: 10.1016/S0260-8774(97)00101-5
[10] ZHENG H, LU H. Effect of microwave pretreatment on the kinetics of ascorbic acid degradation and peroxidase inactivation in different parts of green asparagus (Asparagus officinalis L.) during water blanching[J]. Food Chemistry,2011,128:1087−1093. doi: 10.1016/j.foodchem.2011.03.130
[11] CHANTARO P, DEVAHASTIN S, CHIEWCHAN N. Production of antioxidant high dietary fiber powder from carrot peels[J]. LWT-Food Science and Technology,2008,41:1987−1994. doi: 10.1016/j.lwt.2007.11.013
[12] MAYER-MIEBACH E, SPIESZ W E L. Influence of cold storage and blanching on the carotenoid content of Kintoki carrots[J]. Journal of Food Engineering,2003,56:211−213. doi: 10.1016/S0260-8774(02)00253-4
[13] VORA H M, KYLE W S A, SMALL D M. Activity, localisation and thermal inactivation of deteriorative enzymes in Australian carrot (Daucus carota L.) varieties[J]. Journal of the Science of Food and Agriculture,1999,79:1129−1135. doi: 10.1002/(SICI)1097-0010(199906)79:8<1129::AID-JSFA338>3.0.CO;2-1
[14] NOACH B, DAVID P, AHARON L, et al. Influence of pH treatment on pectic substances and firmness of blanched carrots[J]. Food Chemistry,1992,44:251−254. doi: 10.1016/0308-8146(92)90046-5
[15] LINHARES M D F D, ALVES FILHO E G, SILVA L M A, et al. Thermal and non-thermal processing effect on açai juice composition[J]. Food Research International,2020,136:109506. doi: 10.1016/j.foodres.2020.109506
[16] DICKINSON E. Structure and rheology of simulated gels formed from aggregated colloidal particles[J]. Journal of Colloid and Interface Science,2000,225(1):2−15. doi: 10.1006/jcis.1999.6662
[17] VARALAKSHMI S. A Review on the application and safety of non-thermal techniques on fresh produce and their products[J]. LWT-Food Science and Technology,2021,149:111849. doi: 10.1016/j.lwt.2021.111849
[18] ZHAO L, WANG S, LIU F, et al. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks[J]. Innovative Food Science & Emerging Technologies,2013,17:27−36.
[19] 中华人民共和国国家卫生和计划生育委员会. GB 4789.15-2016 食品安全国家标准 食品微生物学检验 霉菌和酵母菌计数[S]. 北京:中国标准出版社, 2016. [National Health and Family Planning Commission of the People's Republic of China. GB 4789.15-2016 National standards for food safety Microbiology detection Moulds and yeasts count[S]. Beijing:Standards Press of China, 2016.] National Health and Family Planning Commission of the People's Republic of China. GB 4789.15-2016 National standards for food safety Microbiology detection Moulds and yeasts count[S]. Beijing: Standards Press of China, 2016.
[20] 中华人民共和国国家卫生和计划生育委员会, GB 4789.2-2016 国家食品药品监督管理总局. 食品安全国家标准 食品微生物学检验 菌落总数测定[S]. 北京:中国标准出版社, 2016. [National Health and Family Planning Commission of the People's Republic of China, State Food and Drug Administration. GB 4789.2-2016 National standards for food safety Microbiology detection Aerobic plate count[S]. Beijing:Standards Press of China, 2016.] National Health and Family Planning Commission of the People's Republic of China, State Food and Drug Administration. GB 4789.2-2016 National standards for food safety Microbiology detection Aerobic plate count[S]. Beijing: Standards Press of China, 2016.
[21] ZHAO L, QIN X, HAN W, et al. Novel application of CO2-assisted high pressure processing in cucumber juice and apple juice[J]. LWT-Food Science and Technology,2018,96:491−498. doi: 10.1016/j.lwt.2018.06.003
[22] EL-NAKHEL C, PANNICO A, GRAZIANI G, et al. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity[J]. Antioxidants,2020,9(4):300. doi: 10.3390/antiox9040300
[23] 曹建康, 姜微波, 赵玉梅. 果蔬采后生理生化实验指导[M]. 北京:中国轻工业出版社, 2007. [CAO J K, JIANG W B, ZHAO Y M. Physiological and biochemical experiment instruction of postharvest fruits and vegetables[M]. Beijing:China Light Industry Press, 2007.] CAO J K, JIANG W B, ZHAO Y M. Physiological and biochemical experiment instruction of postharvest fruits and vegetables[M]. Beijing: China Light Industry Press, 2007.
[24] 中华人民共和国国家卫生和计划生育委员会. GB 7101-2015 食品安全国家标准 饮料[S]. 北京:中国标准出版社, 2015. [National Health and Family Planning Commission of the People's Republic of China. GB 7101-2015 National food safety standard for beverages[S]. Beijing:China Standards Publishing House, 2015.] National Health and Family Planning Commission of the People's Republic of China. GB 7101-2015 National food safety standard for beverages[S]. Beijing: China Standards Publishing House, 2015.
[25] WU W, XIAO G, YU Y, et al. Effects of high pressure and thermal processing on quality properties and volatile compounds of pineapple fruit juice[J]. Food Control,2021,130:108293. doi: 10.1016/j.foodcont.2021.108293
[26] AAMIR, M, OVISSIPOUR M, RASCO B, et al. Seasonality of the thermal kinetics of color changes in whole spinach (Spinacia oleracea) leaves under pasteurization conditions[J]. International Journal of Food Properties,2014,17(9):2012−2024. doi: 10.1080/10942912.2013.779701
[27] GERALDI M V, BETIM CAZARIN C B, DIAS-AUDIBERT F L, et al. Influence of high isostatic pressure and thermal pasteurization on chemical composition, color, antioxidant properties and sensory evaluation of jabuticaba juice[J]. LWT-Food Science and Technology,2021,139:110548. doi: 10.1016/j.lwt.2020.110548
[28] WANG R, XU Q, YAO J, et al. Post-effects of high hydrostatic pressure on green color retention and related properties of spinach puree during storage[J]. Innovative Food Science & Emerging Technologies,2013,17:63−71.
[29] 郭世豪. 不同加工方式对西兰花茎叶汁风味的影响[D]. 杭州:浙江工商大学, 2020, 89. [GUO S H. Effect of different processing methods on the flavor of Broccoli stem and leaf juice[D]. Hangzhou:Zhejiang University of Technology and Industry, 2020, 89.] GUO S H. Effect of different processing methods on the flavor of Broccoli stem and leaf juice[D]. Hangzhou: Zhejiang University of Technology and Industry, 2020, 89.
[30] 叶田, 张献忠, 秦静, 等. 超高压和巴氏热杀菌处理对鲜榨西芹汁品质影响的比较研究[J]. 饮料工业,2018,21(3):43−46. [YE T, ZHANG X Z, QIN J, et al. Comparative study on the effects of ultra-high pressure and pasteurization of chinese vegetables on the quality of fresh celery juice[J]. Beverage Industry,2018,21(3):43−46.] YE T, ZHANG X Z, QIN J, et al. Comparative study on the effects of ultra-high pressure and pasteurization of chinese vegetables on the quality of fresh celery juice[J]. Beverage Industry, 2018, 21(3): 43−46.
[31] HELENA D O S, FERNANDA C R, GILIANI, et al. Influence of processing conditions on the composition of feijoa (Acca sellowiana) juices during storage[J]. Journal of Food Composition and Analysis,2022,114:104769. doi: 10.1016/j.jfca.2022.104769
[32] WANG R, DING S, HU X, et al. Effects of high hydrostatic pressure on chlorophylls and chlorophyll-protein complexes in spinach[J]. European Food Research and Technology,2016,242(9):1533−1543. doi: 10.1007/s00217-016-2654-8
[33] XU X, DENG J, LUO D, et al. Comparative study of high hydrostatic pressure and high temperature short time processing on quality of clear and cloudy Se-enriched kiwifruit juices[J]. Innovative Food Science & Emerging Technologies,2018,49:1−12.
[34] LEE J, KANG Y, KIM Y J, et al. Effect of high pressure and treatment time on nutraceuticals and antioxidant properties of Lonicera japonica Thunb[J]. Innovative Food Science & Emerging Technologies,2019,54:243−251.
[35] SÁNCHEZ C, BARANDA A B, MARTÍNEZ DE MARAÑÓN I. The effect of high pressure and high temperature processing on carotenoids and chlorophylls content in some vegetables[J]. Food Chemistry,2014,163:37−45. doi: 10.1016/j.foodchem.2014.04.041
[36] 冉露霞, 王俊杰, 成臣, 等. 超高压和巴氏杀菌对百香果汁贮藏期品质的影响[J]. 食品工业科技,2023,44(3):56−66. [RAN L X, WANG J J, CHENG C, et al. Effect of ultra-high pressure sterilization and pasteurization on the quality of passion fruit juice during storage[J]. Science and Technology of Food Industry,2023,44(3):56−66.] RAN L X, WANG J J, CHENG C, et al. Effect of ultra-high pressure sterilization and pasteurization on the quality of passion fruit juice during storage[J]. Science and Technology of Food Industry, 2023, 44(3): 56−66.
[37] FEUMBA DIBANDA R, PANYOO AKDOWA E, RANI P. A, et al. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings[J]. Food Chemistry,2022,302:125308.
[38] VAN LOEY A, OOMS V, WEEMAES C, et al. Thermal and pressure−temperature degradation of chlorophyll in broccoli (Brassica oleracea L. italica) juice: A kinetic study[J]. Journal of Agricultural and Food Chemistry, 1998, 46(12):5289−5294.
[39] HITHAMANI G, MEDAPPA H, CHAKKARAVARTHI A, et al. Effect of adsorbent and acidulants on enzymatic browning of sugarcane juice[J]. Journal of Food Science and Technology,2018,55:4356−4362. doi: 10.1007/s13197-018-3350-4
[40] KAMBLE H A, GATADE A A, SAHOO A K, et al. Effect of blanching treatment on antioxidant activity and color values of sugarcane juice[J]. Materials Today:Proceedings,2021,47:5663−5667. doi: 10.1016/j.matpr.2021.03.706
[41] ZHANG H, BHUNIA K, KUANG P, et al. Effects of oxygen and water vapor transmission rates of polymeric pouches on oxidative changes of microwave-sterilized mashed potato[J]. Food and Bioprocess Technology,2016,9:341−351. doi: 10.1007/s11947-015-1628-3
[42] WANG L, DENG W, WANG P, et al. Degradations of aroma characteristics and changes of aroma related compounds, PPO activity, and antioxidant capacity in sugarcane juice during thermal process[J]. Journal of Food Science,2020,85:1140−1150. doi: 10.1111/1750-3841.15108





 下载:
下载:
 下载:
下载:
