Effect of Polysaccharide Addition on the Stability and Gel Properties of Rabbit Myofibrillar Pickering Emulsion
-
摘要: 本研究提取兔肉肌原纤维蛋白(Rabbit Myofibrillar Protein,RMP),以大豆油为油相制备Pickering乳液及乳液凝胶,考察不同pH环境(3~10)、不同浓度卡拉胶(0.25%~0.50%)和海藻酸钠(0.25%~0.50%)添加对Pickering乳液稳定性及其凝胶特性的影响,以期利用RMP制备高稳定性Pickering乳液凝胶。结果表明:当pH为9时,RMP乳液的ζ-电位绝对值最大(33.60 mV);在pH为9,油相体积50%,卡拉胶和海藻酸钠多糖添加浓度均为0.35%时,RMP-Pickering乳液的ζ-电位绝对值(72.97±0.60)、乳化指数(Emulsifying Activity Index,EAI)(5.09±0.09 m2·g−1)和乳化稳定指数(Emulsifying Stability Index,ESI)(46.07%±3.74%)均达到最大值;在多糖浓度0.25%~0.35%范围内时,卡拉胶和海藻酸钠添加的RMP-Pickering乳液凝胶的持水性、硬度和弹性均逐渐升高,二者均在添加浓度0.35%时达到最大值,且当多糖浓度在0.35%~0.50%范围内时,海藻酸钠-RMP乳液凝胶较卡拉胶-RMP乳液凝胶具有更低的硬度和更高的持水性与弹性;红外光谱分析显示随着多糖浓度的增加,两种多糖稳定的RMP乳液凝胶β-折叠含量均呈现先增加后减少的趋势,蛋白质二级结构由无序变得有序,在两种多糖浓度均为0.35%时,β-折叠含量达到最大值(卡拉胶:30.34%±0.04%,海藻酸钠:29.70%±0.12%);由宏微观结构及凝胶作用力分析可知,疏水相互作用和二硫键均在卡拉胶-和海藻酸钠-RMP Pickering乳液凝胶维持凝胶结构中发挥了作用。综上,当卡拉胶和海藻酸钠的终浓度均为0.35%时,能够使多糖-RMP-Pickering乳液体系分布更加均匀,不易发生聚集,且能够进一步形成具有良好质构特性的乳液凝胶。
-
关键词:
- 兔肉肌原纤维蛋白 /
- 卡拉胶 /
- 海藻酸钠 /
- Pickering乳液 /
- 乳液凝胶
Abstract: In this study, rabbit myofibrillar protein (RMP) was extracted, and Pickering emulsion and the emulsion gel were prepared with soybean oil as the oil phase. The effects of different pH environment (3~10), carrageenan (0.25%~0.50%) and sodium alginate (0.25%~0.50%) on the stability and gel properties of Pickering emulsion were investigated, in order to prepare Pickering emulsion gel with high stability by using RMP. The results showed that when pH value was 9, the absolute value of ζ-potential of RMP emulsion was the largest (33.60 mV). The absolute value of ζ-potential (72.97±0.60), emulsifying activity index (EAI) (5.09±0.09 m2·g−1) and emulsifying stability index (ESI) (46.07%±3.74%) of RMP-Pickering emulsion all reached the maximum value when the oil phase volume was 50% and the concentration of carrageenan and sodium alginate polysaccharide were both 0.35% at pH9. In the range of polysaccharide concentration from 0.25% to 0.35%, the water holding capacity, hardness and elasticity of RMP-Pickering emulsion gel with carrageenan and sodium alginate added all increased gradually, and these indicators reached the maximum value at the polysaccharide concentration of 0.35%. Besides, the sodium alginate-RMP Pickering emulsion gel had lower hardness and higher water holding capacity and elasticity than carrageenan-RMP gel, in the range of polysaccharide concentration from 0.35% to 0.50%. Infrared spectrum analysis showed that, with the increase of polysaccharide concentration, the content of β-folded of the RMP Pickering emulsion gel stabilized by the two polysaccharides increased first and then decreased, and the secondary structure of proteins changed from disordered to ordered. When both concentration of polysaccharides were 0.35%, the β-folded content reached the maximum (carrageenan group: 30.34%±0.04%, sodium alginate group: 29.70%±0.12%). According to analysis of macro and micro structures and gel force, hydrophobic interaction and disulfide bond both played a role in maintaining gel structure of carrageenan- and sodium alginate-RMP Pickering emulsion gel. In conclusion, when the final concentrations of carrageenan and sodium alginate were both 0.35%, the polysaccharides-RMP-Pickering emulsion system could be distributed more evenly, and aggregation was not easy to occur, and the emulsion gel with good texture characteristics could be further formed. -
兔肉蛋白质含量丰富(高达70%),是机体高生物价态氨基酸的优质来源[1]。兔肌原纤维蛋白(Rabbit Myofibrillary Protein,RMP)大致可占总蛋白质的55%~60%,含有8种人体必需的氨基酸,具有盐溶性、凝胶特性、乳化性、起泡性,能够显著影响肉制品的质构、嫩度和保水性等品质[2-4],并形成较为稳定的Pickering乳液凝胶[5-6]。我国是世界兔肉生产第一大国,兔肉产量基本占到了世界兔肉产量的60%[4],而兔肉深加工程度严重不足,产品单一,附加值较低,且消费者接受度不高,这些问题极大限制了兔产业的发展。因此,利用RMP制备高稳定性乳液凝胶能够为功能性健康食品开发提供技术参考和创新依据。
乳液凝胶是指一种被乳状液液滴填充的具有凝胶网状结构,且机械性能较强的凝胶[7-8],可以包埋亲水和疏水成分,保护、缓释、控释生物活性物质,其作为动物脂肪替代物已广泛应用于肉制品、奶酪、酸奶、糕点、冰淇淋等食品加工领域[9-10]。但是,现有乳液凝胶存在稳定性不强、凝胶剂不安全等问题。较之传统小分子表面活性剂,天然大分子胶体颗粒(如多糖、蛋白质、复合物)在制备Pickering乳液凝胶方面,多糖颗粒稳定的蛋白质Pickering乳液能够使液滴聚集,相互作用形成连续的乳液颗粒型凝胶网络结构,显示出稳定性高、安全环保、生物相容性好、可塑性强等优点[11-12]。研究显示,阴离子多糖如卡拉胶、海藻酸钠等多糖生物聚合物也已被证实具有较好的乳化性能,且其表面活性易通过改性进行调节,具有较强的空间稳定性,可以抵抗絮凝和凝聚等现象,从而提高Pickering 乳液凝胶稳定性[13-15]。目前,以RMP为基材,利用多糖生物聚合物制备RMP-Pickering乳液及其凝胶的研究尚未见报道。
本研究制备了RMP凝胶颗粒,并利用该颗粒在一定pH环境下,以大豆油为油相制备Pickering乳液及乳液凝胶,通过考察不同浓度卡拉胶(0.25%~0.50%)和海藻酸钠(0.25%~0.50%)添加对Pickering乳液稳定性及凝胶特性的影响,分析ζ-电位绝对值、动态光散射特性、乳化指数(EAI)和乳化稳定指数(ESI)、持水性、质构、蛋白质二级结构、宏微观结构和凝胶作用力等指标,构建并表征复合多糖稳定的RMP-Pickering乳液及其凝胶体系。
1. 材料与方法
1.1 材料与仪器
冷鲜兔肉(福建肉兔,50~60日龄)、大豆油(金龙鱼有限公司) 购自漳州市大润发超市;氯化钠、磷酸二氢钠、十二烷氨基磺酸钠、乙二醇二乙醚二胺四乙酸(EGTA)、甘氨酸、浓盐酸、柠檬酸、磷酸氢二钠、磷酸二氢钾、氢氧化钠 分析纯,西陇科学股份有限公司;三羟甲基氨基甲烷 分析纯,南京探求生物技术有限公司。
DF-101S集热式恒温加热磁力搅拌器 上海力辰邦西仪器科技有限公司;T25 digital Ultra-turrak均质机 德国IKA集团;DM500生物显微镜 德国徕卡相机股份公司;EX225ZH/AD电子分析天平 奥豪斯仪器(上海)有限公司;NICOLETiSI0傅立叶红外分光光度计 美国赛默飞世尔公司;VC505/VC750超声波细胞破碎仪 美国SONICS & MATERIALS公司;UV5100B紫外可见分光光度计 上海元析仪器有限公司;Mastersize激光粒度仪、ZS90Zeta电位分析仪 马尔文仪器有限公司;CT3-10K质构仪 美国博勒飞公司。
1.2 实验方法
1.2.1 RMP的提取
RMP的提取依照Park等[16]的方法,将冷鲜兔肉(约400 g)洗净绞碎放入提前配制好的约四倍体积的磷酸缓冲液(pH7.2,20 mmol/L)中,溶液中NaCl、MgCl2和乙二胺四乙酸二钠质量浓度分别为0.1、0.1和0.1 mmol/L,之后用均质机均质1 min,均质液在8000 r/min、4 ℃离心17 min。倒掉上清液,按同样比例加入提取液,并重复上述均质和离心步骤3次。之后加入四倍体积20 mmol/L磷酸缓冲液(pH6.25,其中包含0.1 mol/L NaCl),按照上述步骤离心。取出沉淀物,加入100 mL磷酸盐缓冲液(pH6.0)并匀浆。然后用120目过滤袋过滤,滤液在8000 r/min、4 ℃条件下离心17 min,所得沉淀冷冻干燥后即为RMP。
1.2.2 多糖-RMP乳液的制备
1.2.2.1 多糖-RMP乳液的制备方法
参考朱秀清等[17]的方法并稍作修改:将RMP分散溶解于磷酸盐缓冲液(0.02 mol·L−1,pH7.0)中,使得溶液中RMP终浓度为5%(w/v),于25 ℃室温条件下1000 r/min磁力搅拌2 h,确保RMP完全溶解。通过滴加0.01 mol/L HCl或NaOH将分散液pH分别调节至3.0~10.0。向RMP溶液中分别加入不同浓度的海藻酸钠、卡拉胶,使其终浓度为0.25%~0.50%,边添加边搅拌,待形成多糖-RMP复合物后搅拌30 min,随后加入内相体积分数为50%的大豆油,利用高速均质剪切机控温条件下(10 ℃以下)7000 r/min均质2 min,即制得由两种多糖-RMP复合物稳定的Pickering乳液。
1.2.2.2 pH环境对RMP乳液粒径和ζ-电位的影响
将提取的RMP分散在去离子水中,使得终浓度为5%(w/v),于25 ℃室温条件下将乳液pH分别调节为3、4、5、6、7、8、9、10。将乳液稀释500倍测粒径,稀释1000倍测电位。
1.2.2.3 卡拉胶浓度对多糖-RMP Pickering乳液ζ-电位的影响
固定乳液pH9,海藻酸钠终浓度为0.30%,大豆油内相体积分数为50%。考察卡拉胶终浓度依次为0.25%、0.30%、0.35%、0.40%、0.45%、0.50%条件下多糖-RMP Pickering乳液的ζ-电位绝对值变化。
1.2.2.4 海藻酸钠浓度对多糖-RMP Pickering乳液ζ-电位的影响
固定乳液pH为9,卡拉胶终浓度为0.35%,大豆油内相体积分数为50%。考察海藻酸钠终浓度依次为0.25%、0.30%、0.35、0.40%、0.45%、0.50%条件下,多糖-RMP乳液的ζ-电位绝对值变化。
1.2.3 动态光散射测定ζ-电位
根据Khalesi等[18]的方法稍微改动,应用Mastersizer仪器测量乳液颗粒尺寸分布(d4,3)和ζ-电位。将制备好的不同多糖-RMP Pickering乳液分散到0.02 mol·L−1,pH7.0磷酸盐缓冲液中,分别稀释500倍用于测粒径,稀释1000倍用于测电位。
1.2.4 乳化指数(EAI)和乳化稳定指数(ESI)测定
根据Zhang等[19]的方法并稍加改动,测定乳化活性及乳化稳定性。取上述制备的乳液,用0.1%十二烷基磺酸钠(SDS)稀释至500倍,混匀后测吸光度值。以0.1% SDS为空白在波长为500 nm紫外光分光光度计下测量乳液吸光度记为A1;30 min过后在同样波长和以同样空白测吸光度,为A2。EAI和ESI计算公式如下:
式中:N为稀释倍数(500);C为前蛋白质质量浓度(g·mL−1);H为乳化液中油相体积分数,0.5;A1为0 min时的吸光值;A2为30 min时的吸光值。
1.2.5 多糖-RMP Pickering乳液凝胶的制备
在1.2.2基础上,将上述制备的乳液于80 ℃加热20 min,取出后迅速于冰水浴中冷却至室温,于4 ℃冰箱储存备用[20]。
1.2.6 多糖-RMP Pickering乳液凝胶特性测定
1.2.6.1 持水性
参考Boutin等[21]的方法并适当修改。称取乳液凝胶10 g左右放入10 mL离心管中离心,离心条件为4 ℃,1000×g条件下离心15 min后取出,凝胶底部的水倒置放置使得水快速挥发,随后测量离心前和离心后的乳液凝胶和离心管的总重量测定乳液的持水性。
式中:WHC为持水性,%;H1为样品离心后无水分时管和凝胶的重量,g;H2为样品离心前有水分时管和凝胶的重量,g。
1.2.6.2 红外光谱分析(ATR)
采用傅立叶红外分光光度计对乳液蛋白质二级结构进行相关含量的测定,液体采用衰减全反射(ATR)技术,参照Majzner等[22]的方法进行测定,采用红外光谱仪扫描,范围为4000~400 cm−1,分辨率参数选择4 cm−1,测定后的红外光谱图数据进行数据处理后采用PeakFit软件选择1600~1700 cm−1波长对蛋白质二级结构进行二阶求导,以峰面积求得不同结构含量。
1.2.6.3 质构特性测定
按照Zhao等[23]描述的方法并在此基础上略有改动,将制备的多糖-RMP Pickering乳液凝胶在4 °C冰箱内放置过夜,之后取出放于25 ℃室温环境下平衡30 min,用质构仪测定凝胶硬度和弹性。探头为TA/0.5,测前速度1 mm·s−1,测定速度1 mm·s−1,测后速度1 mm·s−1,触发力为1 g,测定距离为凝胶高度1/2。
1.2.6.4 微观结构观察
采用光学显微镜观察乳液凝胶的微观结构。用磷酸盐缓冲液将样品稀释一定倍数后放置在载玻片上,采用光学显微镜于物镜40×、目镜10×下进行观察。
1.2.6.5 凝胶作用力
根据Tang等[24]的方法稍加改动,研究不同多糖0.35%添加量下乳液凝胶作用力。将乳液凝胶溶于以下四种不同溶液,A液:蒸馏水;B液:pH8.0的缓冲溶液(其中含有121.14 g/mol Tris,75.07 g/mol甘氨酸,372.24 g/mol EDTA-2 Na);C液:B液+20 g/L十二烷氨基磺酸钠;D液:C液+10 g/L β-硫基乙醇。将1 g乳液凝胶放入15 mL以上四种不同溶液中,搅拌1 h,分散液在6830 r/min,4 ℃条件下离心15 min,离心后取上层乳液经相应溶液稀释30倍后在600 nm下测吸光度值,计算浊度值,浊度值公式如下。
式中:T为每cm浊度,cm−1;A为600 nm下吸光度值;S为稀释倍数;L为容器光程长度,cm。
1.3 数据处理
实验平行测定三次,结果用平均值±标准偏差表示;利用SPSS Statistics 24软件对数据进行ANOVA和Duncan检验统计分析;以P<0.05表示差异显著;利用Origin 2018进行绘图。
2. 结果与分析
2.1 pH环境对RMP乳液动态光散射的影响
通过动态光散射测定不同pH环境条件下RMP乳液的粒径及电位,以表征乳液的稳定性。在pH4和5时,RMP乳液为不稳定的悬浊液,形成了肉眼可见的聚集体,仪器只能粗略测得该pH环境条件下RMP乳液中聚集体的平均粒径约为3972.5~4708.6 nm和3966.2~4683.9 nm。RMP乳液的平均粒径随环境pH变化显著(P<0.05)。当环境pH为7~10时,乳液平均粒径呈现显著增大趋势(P<0.05),且在碱性环境下,RMP颗粒之间相互排斥而导致粒径变大[6,25],且RMP是一种微凝胶颗粒,具有一定的吸水溶胀能力[26]。
如表1所示,RMP乳液ζ-电位绝对值随pH增加而显著变化(P<0.05)。在pH为3时,乳液的ζ-电位绝对值为+5.19,主要是因为RMP外表带入了正电的氨基造成,当pH大于4.5时,乳液的ζ-电位为负值,此时由于乳液中RMP羧基的去质子作用,使得乳液带负电[20]。RMP乳液的ζ-电位绝对值随着pH增加而增加,在碱性条件下逐渐趋于稳定,且在pH为9时达到最大值(33.60)。故确定最适环境pH为9。
表 1 pH环境对RMP乳液粒径和ζ-电位的影响Table 1. Effects of pH on particle size and ζ-potential of RMP emulsion表 2 卡拉胶添加对RMP Pickering乳液粒径和ζ-电位的影响Table 2. Effects of carrageenan addition on particle size and ζ-potential of RMP Pickering emulsion多糖终浓度(%) 粒径(nm) ζ-电位绝对值(mV) 0.25 4391.23±0.21f 65.57±0.47c 0.30 5374.13±0.15e 66.80±0.10b 0.35 6390.17±0.29d 72.77±0.72a 0.40 6401.23±0.25c 63.83±0.41d 0.45 6564.63±0.32b 62.77±0.68d 0.50 7208.12±0.10a 60.93±0.90e 表 3 海藻酸钠添加对RMP Pickering乳液粒径和ζ-电位的影响Table 3. Effects of sodium alginate addition on particle size and ζ- potential of RMP Pickering emulsion多糖终浓度(%) 粒径(nm) ζ-电位绝对值(mV) 0.25 6400.20±0.20f 69.30±0.56b 0.30 6500.13±0.15e 71.30±1.01a 0.35 6564.63±0.32d 72.97±0.60a 0.40 6799.17±0.15c 68.77±0.91b 0.45 7152.27±0.31b 56.97±0.61c 0.50 7208.10±0.10a 51.53±1.21d 表 4 不同多糖对RMP Pickering乳液凝胶蛋白质二级结构的影响(%)Table 4. Effects of different polysaccharides on the secondary structure of RMP Pickering emulsion gel protein (%)多糖种类 多糖浓度(%) α-螺旋(%) β-折叠(%) β-转角(%) 无规则卷曲(%) 卡拉胶 0.25 25.51±0.03b 23.46±0.10c 24.75±0.10d 26.28±0.07c 0.30 24.77±0.02c 25.70±0.02b 22.19±0.02e 27.35±0.01b 0.35 23.22±0.05d 30.34±0.04a 21.45±0.14f 28.99±0.10a 0.40 25.61±0.06b 23.15±0.07d 25.43±0.08b 25.80±0.02e 0.45 25.75±0.02a 22.75±0.03e 25.31±0.01c 26.18±0.02d 0.50 25.85±0.07a 22.46±0.04f 25.90±0.08a 25.80±0.06e 海藻酸钠 0.25 24.12±0.01c 27.63±0.16c 21.56±0.09d 26.68±0.05c 0.30 23.64±0.04d 29.07±0.05b 20.49±0.24e 26.80±0.02b 0.35 22.43±0.03e 29.70±0.12a 19.93±0.16f 27.93±0.03a 0.40 24.64±0.03b 26.09±0.01d 22.73±0.02c 26.55±0.01d 0.45 24.69±0.04b 25.93±0.04e 23.19±0.05b 26.19±0.02f 0.50 25.08±0.05a 24.75±0.02f 23.75±0.06a 26.41±0.06e 表 5 不同浓度多糖-RMP Pickering乳液凝胶质构Table 5. Gel texture of different concentrations of polysaccharides-RMP Pickering emulsion多糖 浓度(%) 硬度(g) 弹性(mm) 卡拉胶 0.25 3.15±0.01e 2.46±0.19d 0.30 3.66±0.02c 3.15±0.09c 0.35 6.11±0.01a 3.68±0.05a 0.40 4.59±0.01b 3.48±0.03b 0.45 3.57±0.01d 1.85±0.01e 0.50 3.06±0.01f 1.82±0.01e 海藻酸钠 0.25 2.04±0.02f 1.23±0.02d 0.30 3.36±0.07c 2.16±0.08b 0.35 6.12±0.02a 3.69±0.06a 0.40 3.84±0.01b 2.18±0.01b 0.45 3.18±0.03d 2.11±0.02c 0.50 2.85±0.05e 2.08±0.02c 2.2 多糖对RMP Pickering乳液动态光散射的影响
2.2.1 卡拉胶添加对RMP Pickering乳液动态光散射的影响
如表2所示,乳液粒径大小随卡拉胶终浓度的增加而显著增大(P<0.05),这是因为多糖浓度增加后,乳液变得更加粘稠,流动性减弱,使得乳液粒径变大[17]。同时,ζ-电位绝对值呈现先显著增大后显著降低的趋势(P<0.05)。这可能是因为卡拉胶是阴离子多糖,加入带负电荷多糖-RMP乳液中二者相互排斥,导致电位绝对值明显降低,d4,3略增加[15]。当卡拉胶终浓度为0.35%时,ζ-电位绝对值最大,乳液稳定性最好。
2.2.2 海藻酸钠添加对RMP Pickering乳液动态光散射的影响
如表3所示,固定卡拉胶终浓度为0.35%,当海藻酸钠添加的终浓度为0.25%~0.50%时,乳液体系的粒径呈现显著升高趋势(P<0.05),这是因为海藻酸钠的加入明显提升了乳液的粘稠度[15],乳液流动性大大减弱,粒径显著增大,最高达7208.12 nm,与Pikabea等[26]和江连洲等[20]的研究结果一致。同时,随着海藻酸钠添加量的增加,乳液ζ-电位绝对值呈现先显著升高后显著下降的趋势(P<0.05),当海藻酸钠终浓度0.35%时,ζ-电位绝对值有较大值(72.97 mV),多糖-RMP乳液体系中粒子间斥力增加,此时乳液的稳定性较优。
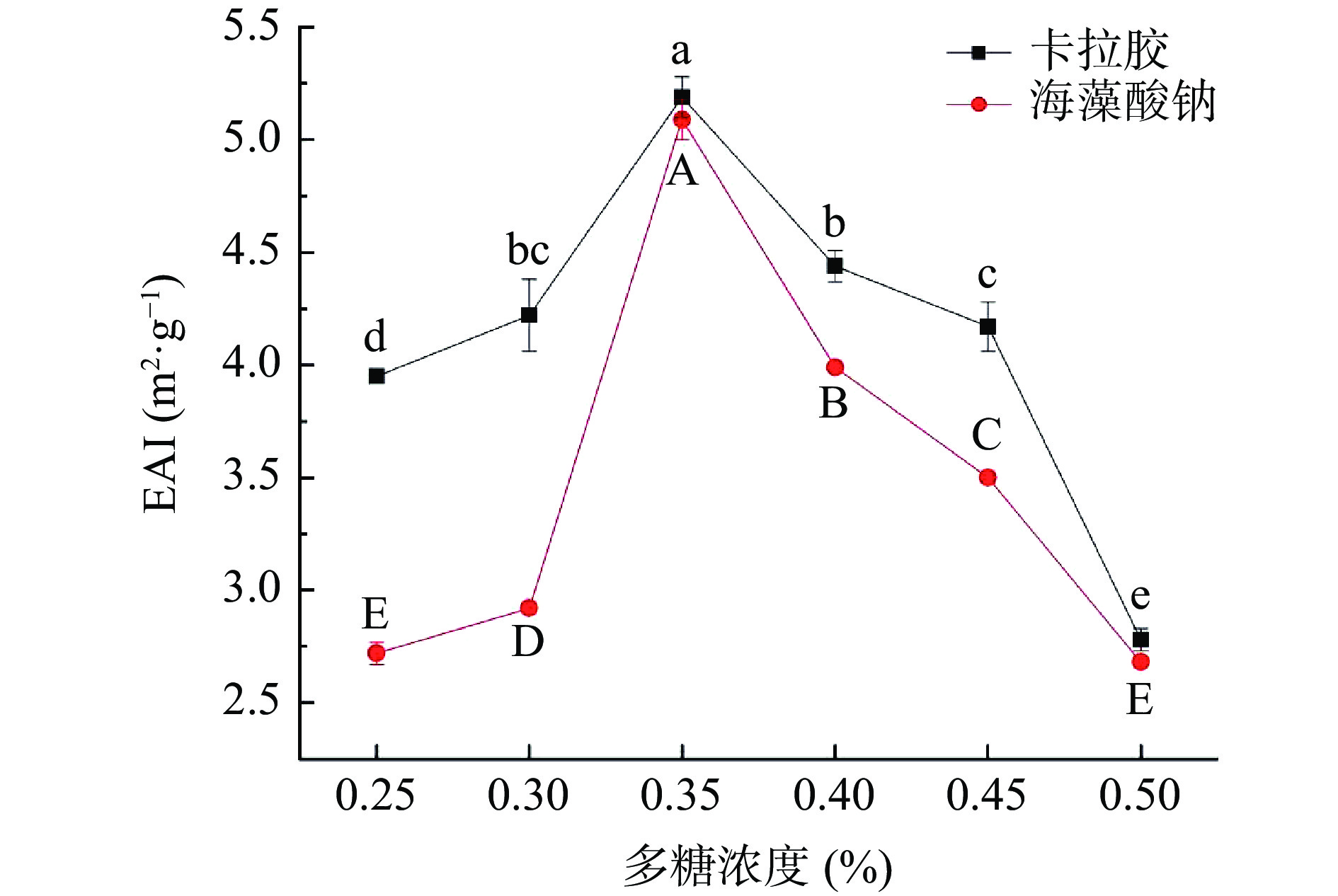
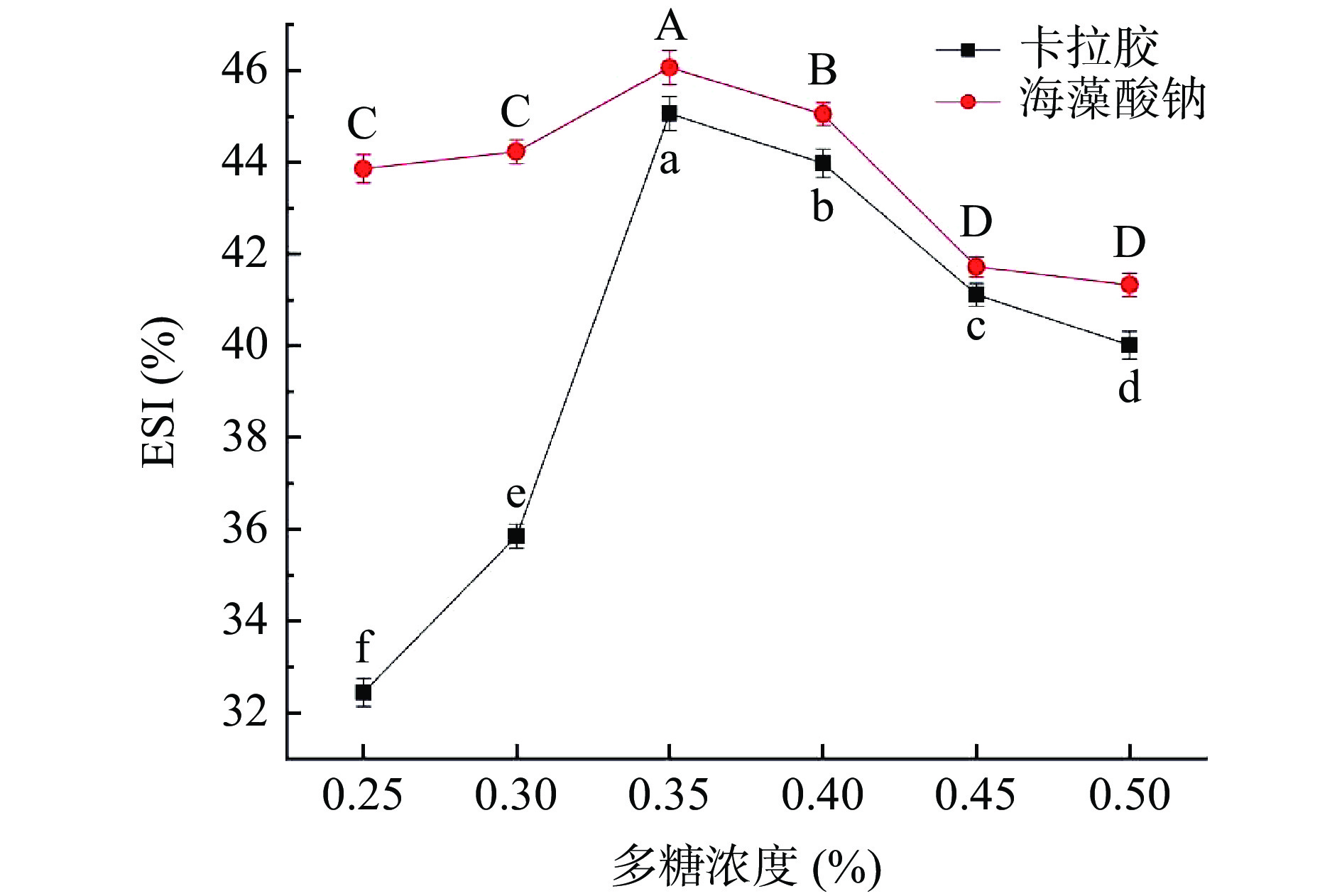
2.3 多糖添加对RMP Pickering乳液凝胶EAI及ESI的影响
如图1和图2可知,卡拉胶和海藻酸钠均可改善RMP Pickering乳液凝胶的乳化性和乳化稳定性。在卡拉胶和海藻酸钠终浓度0.25%~0.50%范围内,随着多糖浓度的增加,乳液凝胶的EAI呈现先升高后下降的趋势(P<0.05),当多糖终浓度为0.35%时,凝胶单位质量乳液乳化表面积(EAI)最大,且卡拉胶添加较之海藻酸钠的EAI更高,乳化性更好。
同样的,随着多糖终浓度的升高,乳化液稳定系数(ESI)也呈现先升高后降低的趋势(P<0.05)。当在卡拉胶和海藻酸钠终浓度均为0.35%时,ESI有最大值,且海藻酸钠的ESI整体较卡拉胶更高。推测出现这样现象的原因可能是,Pickering乳液凝胶形成中,RMP二级结构在加热情况下会改变,发生分子重排,疏水基团充分暴露,同时,随着多糖浓度的增加,RMP与乳液油水界面接触面积逐渐增大,进而使得Pickering乳液乳化性增强[13]。这与Pickering粒子浓度充足时可稳定更大的油水界面,从而形成总表面积较大的小液滴的研究结果一致[27-28]。当多糖浓度进一步升高,多糖会与RMP在油水界面产生竞争吸附作用,又使得RMP在此界面吸附作用减弱,乳化性相应也减弱[13]。卡拉胶和海藻酸钠都属于阴离子多糖,卡拉胶能够随体系温度升高形成可逆凝胶,而海藻酸钠本身不能形成凝胶,需要通过酸化或者钙离子引入形成凝胶,但自身增稠效果显著。凝胶颗粒表面同性电荷越多,相互之间的排斥力使得由该凝胶颗粒组装的Pickering乳液凝胶体系更加趋于稳定,不容易发生絮凝和聚集[11,15]。
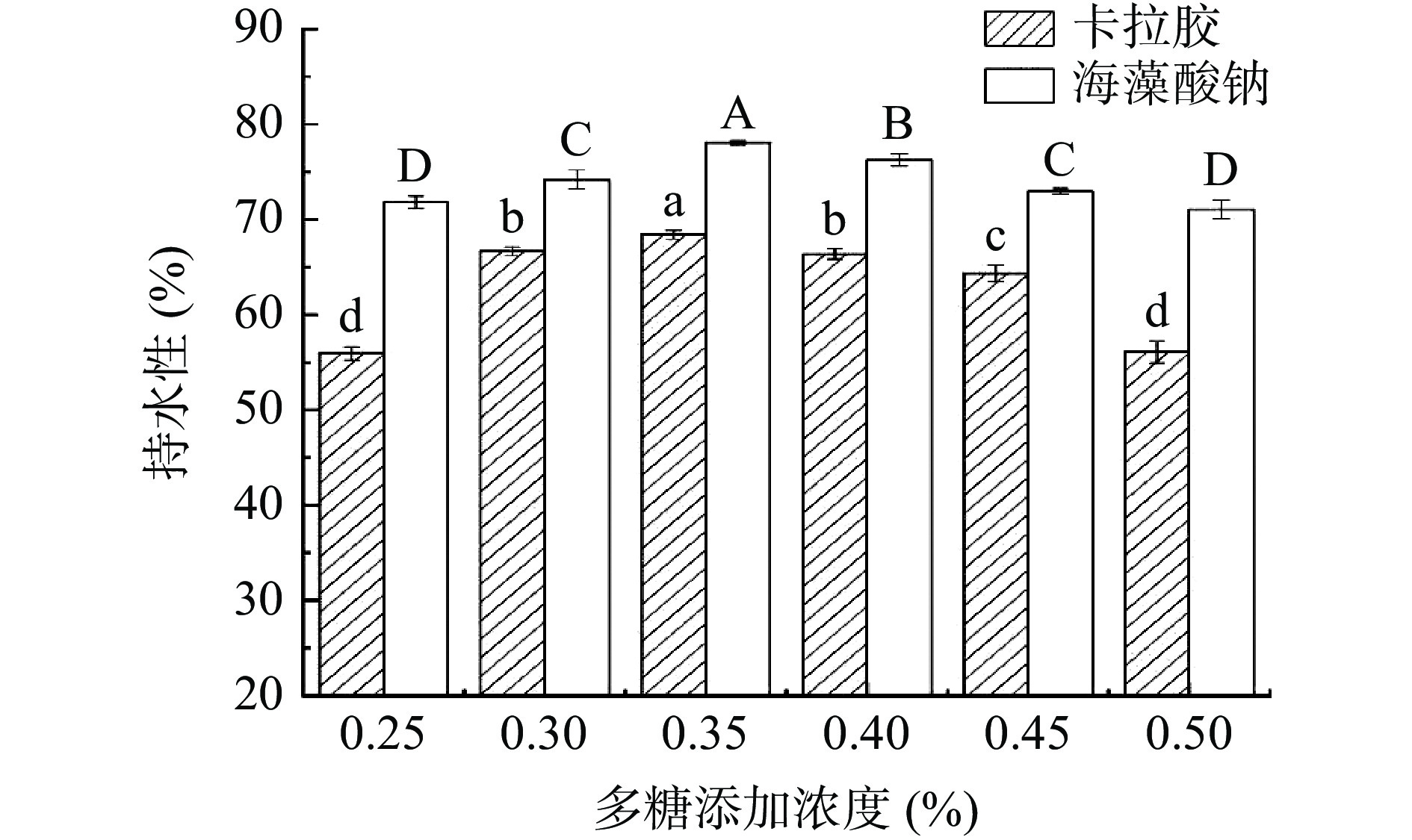
2.4 多糖添加对RMP Pickering乳液凝胶持水性的影响
卡拉胶和海藻酸钠添加对RMP Pickering乳液凝胶持水性的影响如图3所示,当卡拉胶、海藻酸钠多糖终浓度处于0.25%~0.35%范围,随着多糖添加量的增加,凝胶的持水性显著增大(P<0.05),持水性分别达到最大值68.40%和78.07%,当多糖浓度继续增加,凝胶的持水性又显著下降(P<0.05)。这是因为卡拉胶和海藻酸钠能够与RMP产生协同效应,稳定的乳液凝胶网络结构能够截留更多的水分,而当多糖添加量进一步增加,多糖可能会通过水合作用形成连续弱凝胶,或与RMP形成互穿结构,阻碍RMP在后续加热中的交联,导致网络水分渗出增加,持水能力较低[29],此推断与Kuhun等[30]和任菲[31]的研究结果具有一致性。
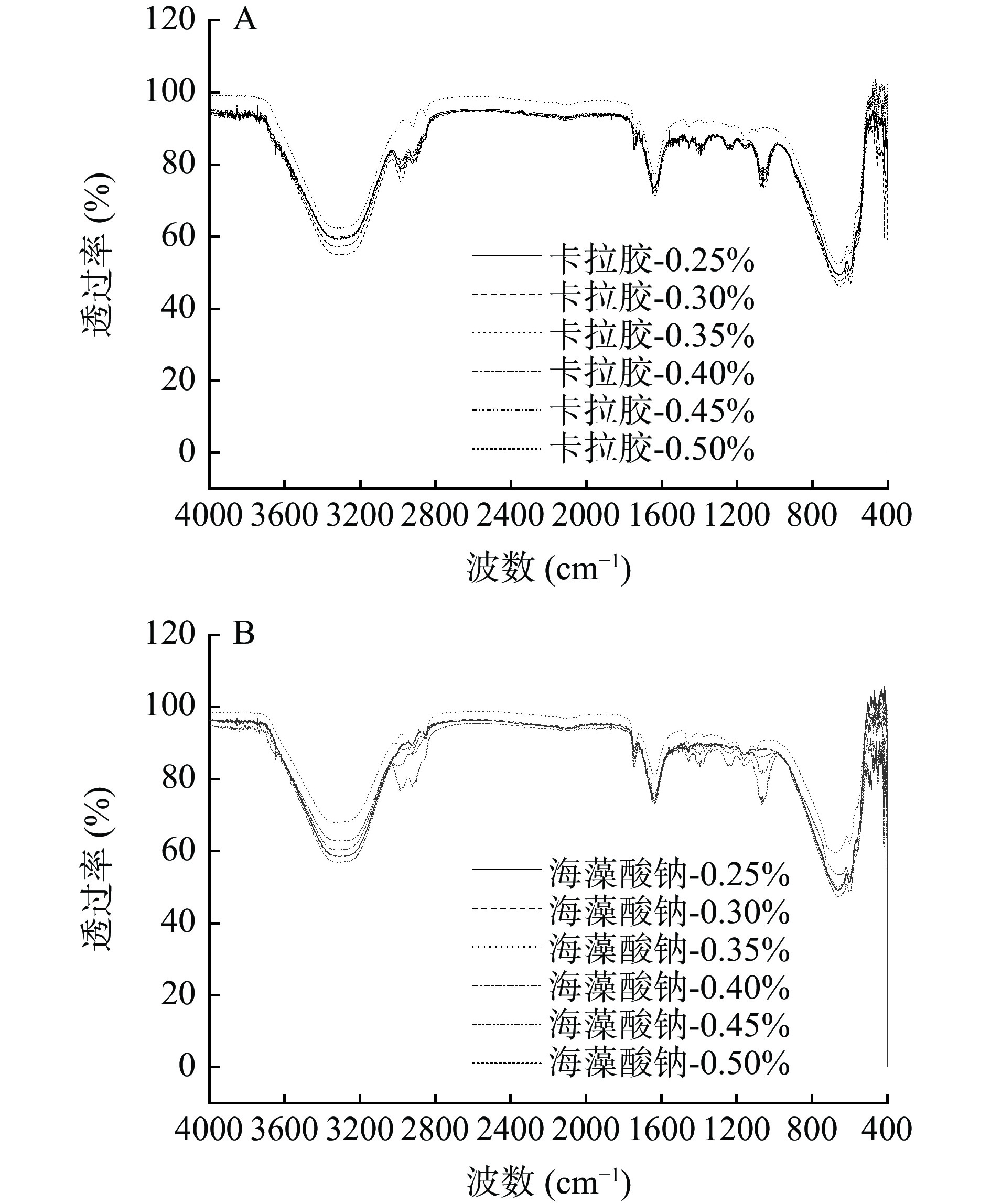
2.5 多糖-RMP Pickering乳液凝胶红外光谱分析(ATR)
通过红外光谱分析表征不同卡拉胶和海藻酸钠稳定的RMP Pickering乳液凝胶RMP二级结构变化,利用Peak Fit拟合软件分析计算图谱(图4),得到不同凝胶中RMP二级结构含量变化(表4)。
由图4可知,不同多糖-RMP Pickering乳液凝胶中RMP存在典型的蛋白质特性峰,即1600~700 cm−1的酰胺Ⅰ区和1230~1320 cm−1的酰胺Ⅲ区[6]。酰胺I带主要由C=O振动引起,与蛋白质肽链的骨架有序程度紧密相关,常用来分析蛋白质(α-螺旋、β-折叠、β-转角和无规卷曲)四种空间结构的相对含量变化[32-33]。从表4可知,随着卡拉胶浓度和海藻酸钠终浓度的增大,α-螺旋和β-转角会呈现先下降后上升的趋势,β-折叠和无规卷曲整体呈现先升高后下降的趋势,当卡拉胶添加量为0.35%时,β-折叠有最大值(30.34%)。β-折叠的含量与蛋白分子聚集程度有关[6],当多糖浓度0.25%~0.35%时,β-折叠增多显著增加了蛋白的柔性结构,同时,凝胶化的热处理过程可能会暴露更多的氨基酸侧链,增强RMP的相互作用,并破坏RMP的α-螺旋的链内氢键,促进α-螺旋向β-折叠结构转变[34],此时,RMP分子由无序变为有序,提升了凝胶的乳化性和稳定性,与前文2.3测定结果一致。
2.6 多糖添加对RMP Pickering乳液凝胶微观结构的影响
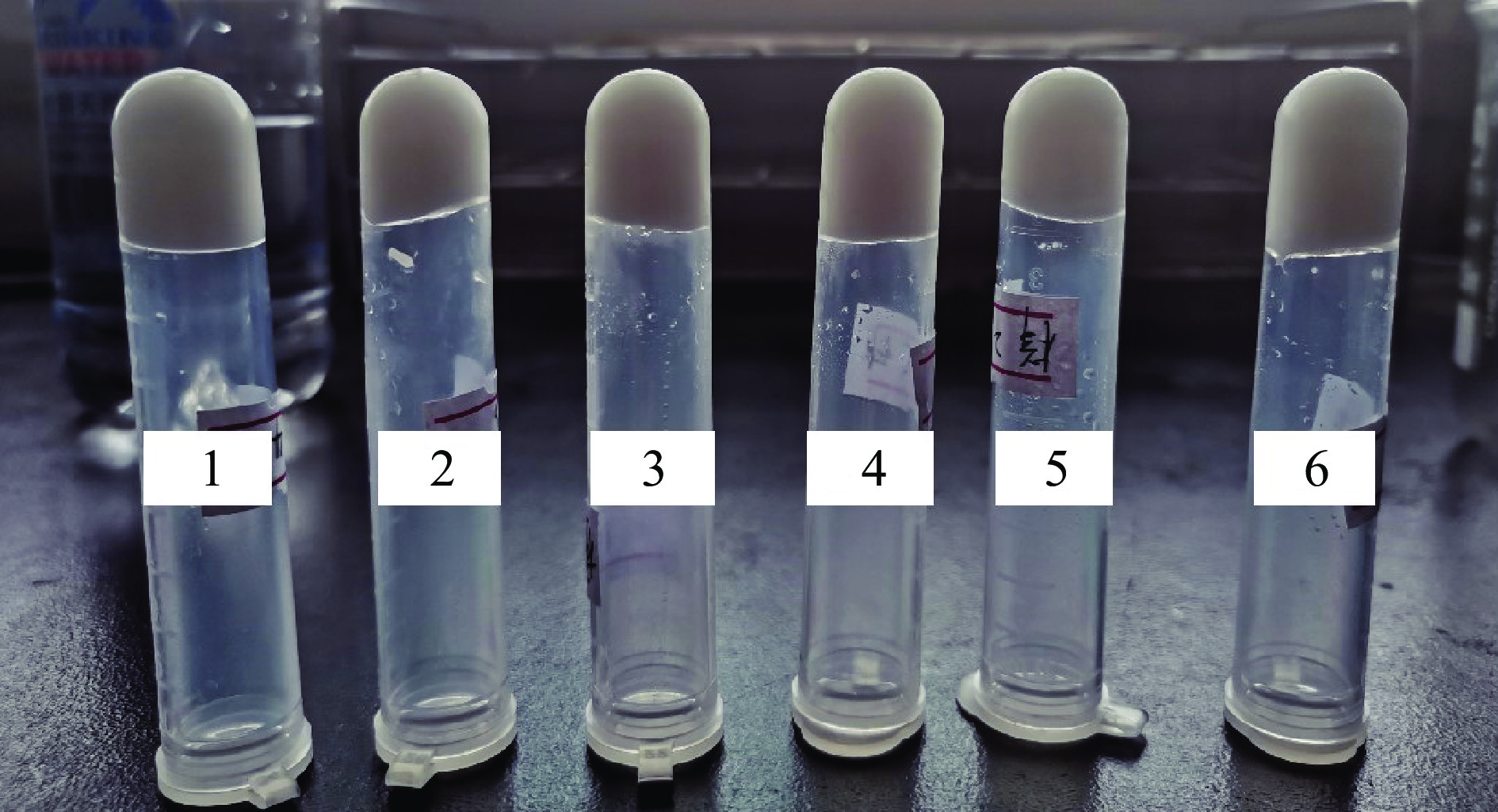
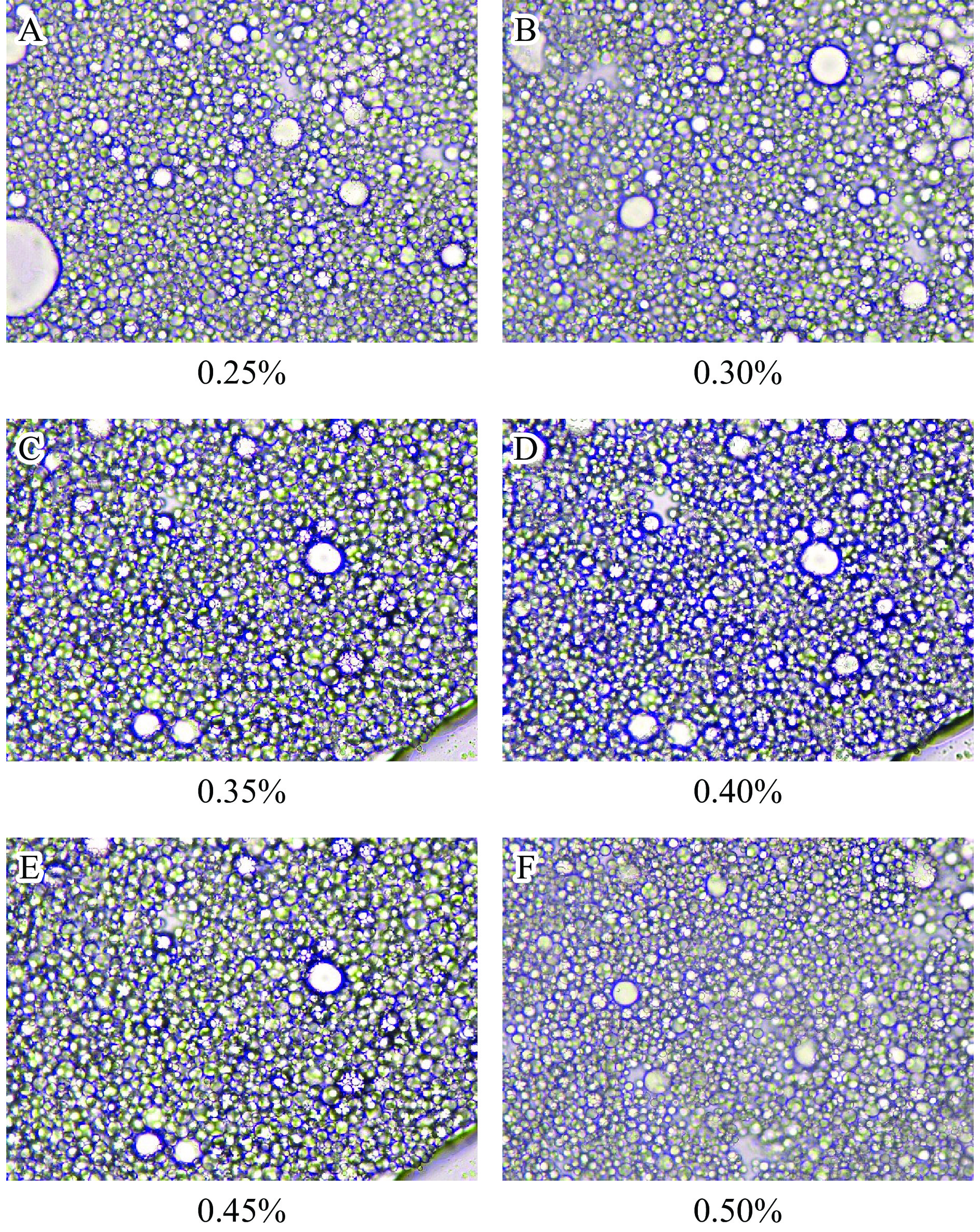
当pH为9环境下,油相体积50%,卡拉胶添加浓度为0.35%时,海藻酸钠添加浓度为0.25%~0.50%时,多糖-RMP Pickering乳液凝胶的外观及微观结构如图5和图6所示。从图5可知,乳液凝胶倒置均未见流动,没有出现油相析出和相分离行为,说明其热稳定性整体较好。不同浓度多糖-RMP Pickering乳液凝胶的油滴粒径基本均匀,当海藻酸钠添加量为0.35%时,油滴粒径更小,排列更紧密(图6)。研究显示,一定量多糖添加至蛋白质中可以提高凝胶的稳定性[35-36],拥挤的油滴会限制油滴的运动,降低其在凝胶化过程中合并析出的可能性,从而提升乳液凝胶稳定性[37]。
![]() 图 5 不同浓度多糖对RMP Pickering乳液凝胶表观图像的影响注:1~6号样品分别为海藻酸钠浓度0.25%、0.30%、0.35%、0.40%、0.45%、0.50%;其他条件:pH9.0,油相体积为50%,卡拉胶浓度为0.35%;图6同。Figure 5. Effects of different concentrations of polysaccharides on the appearance of RMP Pickering emulsion gel
图 5 不同浓度多糖对RMP Pickering乳液凝胶表观图像的影响注:1~6号样品分别为海藻酸钠浓度0.25%、0.30%、0.35%、0.40%、0.45%、0.50%;其他条件:pH9.0,油相体积为50%,卡拉胶浓度为0.35%;图6同。Figure 5. Effects of different concentrations of polysaccharides on the appearance of RMP Pickering emulsion gel2.7 多糖添加对RMP Pickering乳液凝胶质构特性的影响
质构(硬度、弹性等)是表征乳液凝胶特性的重要参数[38-39]。如表5所示,乳液凝胶硬度随卡拉胶、海藻酸钠终浓度增加呈现先增大后减小的趋势,在多糖浓度0.25%~0.35%范围内,两种多糖的硬度和弹性值显著升高(P<0.05),在浓度为0.35%时均达到最大值,分别为6.11±0.01 g,3.68±0.05 mm和6.12±0.02 g,3.69±0.06 mm。这可能是因为在较低浓度多糖添加量(0.25%~0.35%)条件下,多糖与RMP呈互斥状态,由于体系排阻作用使卡拉胶和海藻酸钠对凝胶增效,多糖的加入能够固定更多的水分,促进凝胶形成,提高凝胶强度[40-41]。但是当多糖浓度进一步升高,体系可能因排斥作用而产生絮凝,致使凝胶硬度及弹性又逐渐降低,这与Tang等[42]研究结果类似。
此外,当多糖浓度为0.35%~0.50%范围内,海藻酸钠-RMP凝胶较卡拉胶-RMP凝胶具有较低的硬度和更高的弹性(卡拉胶组的硬度:3.06~6.11 g,弹性:1.82~3.68 mm;海藻酸钠组的硬度:2.85~6.12 g,弹性:2.08~3.69 mm),这可能是由于海藻酸钠可通过增加体系黏度,在体系凝胶化过程中吸附了较多的水分所致[10]。诸多研究也显示,海藻酸钠能够与蛋白类物质复合形成具有特定结构的乳液凝胶,并大大提升包封物的稳定性[37,42]。综上,适量浓度多糖能够促使包裹油滴的蛋白质层厚度增加,抑制油滴聚集,同时调节蛋白凝胶的网络结构,从而改变凝胶质构特性。
2.8 多糖添加对RMP Pickering乳液凝胶作用力的影响
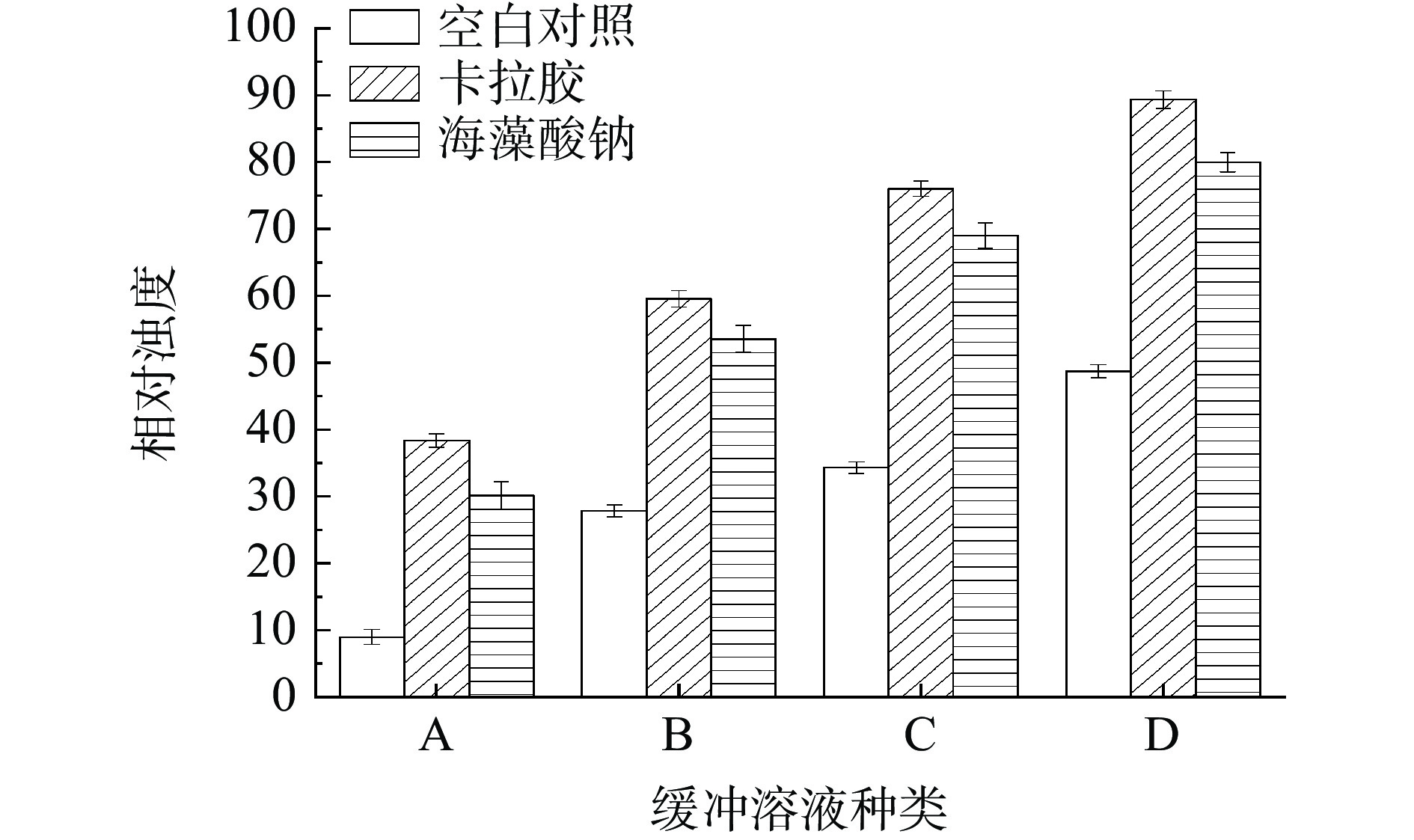
由图7可知,卡拉胶-RMP Pickering乳液凝胶和海藻酸钠-RMP Pickering乳液凝胶在A、B、C、D四种溶液中的溶解度为:D>C>B>A,且卡拉胶组的相对浊度值整体高于海藻酸钠组。推测原因可能是,卡拉胶的添加能够提高乳液凝胶的可塑性、持水性、物理稳定性、硬度和粘弹性等[43]。就卡拉胶-RMP Pickering乳液凝胶在不同溶液中的相对浊度变化来看,C>B说明疏水相互作用在维持凝胶结构中发挥了作用,D>C说明二硫键也在凝胶结构中发挥了作用。同理,疏水相互作用和二硫键也在海藻酸钠-RMP Pickering乳液凝胶维持凝胶结构中发挥了作用。乳液凝胶在各缓冲溶液中溶解度差值都证实了两种多糖加入增强了各种作用力及凝胶强度[17]。
3. 结论
当体系pH9,油相体积50%,不同浓度卡拉胶和海藻酸钠添加对Pickering乳液稳定性及其凝胶特性影响显著。当两种多糖的浓度均为0.35%时,Pickering乳液的ζ-电位绝对值(72.97±0.60)、EAI(5.09±0.09 m2·g−1)和ESI(46.07%±3.74%),以及多糖-RMP-Pickering乳液凝胶的持水性、硬度和弹性均达到最大值,当多糖浓度为0.35%~0.50%范围内,海藻酸钠组较卡拉胶组RMP Pickering乳液凝胶具有较低的硬度和更高的持水性与弹性(卡拉胶组的硬度:3.06~6.11 g,持水性:56.1%~68.4%,弹性:1.82~3.68 mm;海藻酸钠组的硬度:2.85~6.12 g,持水性:71.07%~78.07%,弹性:2.08~3.69 mm)。随着多糖浓度的增大,由两种多糖-RMP稳定的乳液凝胶的β-折叠含量呈现先增加后减少的趋势,蛋白质二级结构由无序变得有序,当两种多糖均为0.35%时,β-折叠含量达到最大值(卡拉胶:30.34%±0.04%,海藻酸钠:29.70%±0.12%),且随着凝胶化的进行,疏水相互作用和二硫键结构形成,凝胶作用力增强。综上,卡拉胶和海藻酸钠添加都能够使RMP-Pickering乳液体系分布更加均匀,当二者终浓度均为0.35%时,能够形成具有良好质构特性的Pickering乳液及其凝胶。
-
图 5 不同浓度多糖对RMP Pickering乳液凝胶表观图像的影响
注:1~6号样品分别为海藻酸钠浓度0.25%、0.30%、0.35%、0.40%、0.45%、0.50%;其他条件:pH9.0,油相体积为50%,卡拉胶浓度为0.35%;图6同。
Figure 5. Effects of different concentrations of polysaccharides on the appearance of RMP Pickering emulsion gel
表 1 pH环境对RMP乳液粒径和ζ-电位的影响
Table 1 Effects of pH on particle size and ζ-potential of RMP emulsion
表 2 卡拉胶添加对RMP Pickering乳液粒径和ζ-电位的影响
Table 2 Effects of carrageenan addition on particle size and ζ-potential of RMP Pickering emulsion
多糖终浓度(%) 粒径(nm) ζ-电位绝对值(mV) 0.25 4391.23±0.21f 65.57±0.47c 0.30 5374.13±0.15e 66.80±0.10b 0.35 6390.17±0.29d 72.77±0.72a 0.40 6401.23±0.25c 63.83±0.41d 0.45 6564.63±0.32b 62.77±0.68d 0.50 7208.12±0.10a 60.93±0.90e 表 3 海藻酸钠添加对RMP Pickering乳液粒径和ζ-电位的影响
Table 3 Effects of sodium alginate addition on particle size and ζ- potential of RMP Pickering emulsion
多糖终浓度(%) 粒径(nm) ζ-电位绝对值(mV) 0.25 6400.20±0.20f 69.30±0.56b 0.30 6500.13±0.15e 71.30±1.01a 0.35 6564.63±0.32d 72.97±0.60a 0.40 6799.17±0.15c 68.77±0.91b 0.45 7152.27±0.31b 56.97±0.61c 0.50 7208.10±0.10a 51.53±1.21d 表 4 不同多糖对RMP Pickering乳液凝胶蛋白质二级结构的影响(%)
Table 4 Effects of different polysaccharides on the secondary structure of RMP Pickering emulsion gel protein (%)
多糖种类 多糖浓度(%) α-螺旋(%) β-折叠(%) β-转角(%) 无规则卷曲(%) 卡拉胶 0.25 25.51±0.03b 23.46±0.10c 24.75±0.10d 26.28±0.07c 0.30 24.77±0.02c 25.70±0.02b 22.19±0.02e 27.35±0.01b 0.35 23.22±0.05d 30.34±0.04a 21.45±0.14f 28.99±0.10a 0.40 25.61±0.06b 23.15±0.07d 25.43±0.08b 25.80±0.02e 0.45 25.75±0.02a 22.75±0.03e 25.31±0.01c 26.18±0.02d 0.50 25.85±0.07a 22.46±0.04f 25.90±0.08a 25.80±0.06e 海藻酸钠 0.25 24.12±0.01c 27.63±0.16c 21.56±0.09d 26.68±0.05c 0.30 23.64±0.04d 29.07±0.05b 20.49±0.24e 26.80±0.02b 0.35 22.43±0.03e 29.70±0.12a 19.93±0.16f 27.93±0.03a 0.40 24.64±0.03b 26.09±0.01d 22.73±0.02c 26.55±0.01d 0.45 24.69±0.04b 25.93±0.04e 23.19±0.05b 26.19±0.02f 0.50 25.08±0.05a 24.75±0.02f 23.75±0.06a 26.41±0.06e 表 5 不同浓度多糖-RMP Pickering乳液凝胶质构
Table 5 Gel texture of different concentrations of polysaccharides-RMP Pickering emulsion
多糖 浓度(%) 硬度(g) 弹性(mm) 卡拉胶 0.25 3.15±0.01e 2.46±0.19d 0.30 3.66±0.02c 3.15±0.09c 0.35 6.11±0.01a 3.68±0.05a 0.40 4.59±0.01b 3.48±0.03b 0.45 3.57±0.01d 1.85±0.01e 0.50 3.06±0.01f 1.82±0.01e 海藻酸钠 0.25 2.04±0.02f 1.23±0.02d 0.30 3.36±0.07c 2.16±0.08b 0.35 6.12±0.02a 3.69±0.06a 0.40 3.84±0.01b 2.18±0.01b 0.45 3.18±0.03d 2.11±0.02c 0.50 2.85±0.05e 2.08±0.02c -
[1] 何琪, 董怡, 邓莎, 等. NaCl对兔肉蛋白质氧化的影响[J]. 中国调味品,2022,47(11):13−16,23. [HE Q, DONG Y, DENG S, et al. Effect of NaCl on protein oxidation of rabbit meat[J]. China Condiment,2022,47(11):13−16,23. HE Q, DONG Y, DENG S, et al. Effect of NaCl on protein oxidation of rabbit meat[J]. China Condiment, 2022, 47(11): 13-16, 23.
[2] 薛山. 我国兔产业发展现状及趋势展望[J]. 肉类研究,2016,30(8):44−48. [XUE S. Present status and future trends in the development of rabbit industry in China[J]. Meat Research,2016,30(8):44−48. doi: 10.15922/j.cnki.rlyj.2016.08.009 XUE S. Present status and future trends in the development of rabbit industry in China[J]. Meat Research, 2016, 30(8): 44-48. doi: 10.15922/j.cnki.rlyj.2016.08.009
[3] ZOTTE A D, SZENDRŐ Z. The role of rabbit meat as functional food[J]. Meat Science,2011,88(3):319−331. doi: 10.1016/j.meatsci.2011.02.017
[4] LI S, ZENG W, LI R, et al. Rabbit meat production and processing in China[J]. Meat Science,2018,145:320−328. doi: 10.1016/j.meatsci.2018.06.037
[5] CHOI Y M, KIM B C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality[J]. Livestock Science,2009,122:105−118. doi: 10.1016/j.livsci.2008.08.015
[6] 裴志胜, 冯紫蓝, 王会博, 等. 金鲳鱼肌原纤维蛋白乳液凝胶的制备及表征[J]. 食品工业科技,2023,44(6):1−17. [PEI Z S, FENG Z L, WANG H B, et al. Fabrication and characterization of high internal phase pickering emulsion manipulated by gel particles of soy protein[J]. Food Industry Science and Technology,2023,44(6):1−17. doi: 10.13386/j.issn1002-0306.2022050308 PEI Z S, FENG Z L, WANG H B, et al. Fabrication and characterization of high internal phase pickering emulsion manipulated by gel particles of soy protein[J]. Food Industry Science and Technology, 2023, 44(6): 1-17. doi: 10.13386/j.issn1002-0306.2022050308
[7] ZHANG B, MENG R, LI X L, et al. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin[J]. Food Chemistry,2021(1):129726.
[8] 崔梦楠, 鹿瑶, 高彦祥, 等. 食品乳液凝胶的制备及其应用研究进展[J]. 食品科学,2019,40(3):323−329. [CUI M N, LU Y, GAO Y X, et al. A review on the preparation and application of food emulsion gels[J]. Food Science,2019,40(3):323−329. doi: 10.7506/spkx1002-6630-20171222-282 CUI M N, LU Y, GAO Y X, et al. A review on the preparation and application of food emulsion gels[J]. Food Science, 2019, 40(3): 323-329. doi: 10.7506/spkx1002-6630-20171222-282
[9] 刁小琴, 李曦, 孙薇婷, 等. 乳液凝胶的构建及应用研究进展[J]. 食品安全质量检测学报,2022,13(4):1036−1043. [DIAO X Q, LI X, SUN W T, et al. Research progress in the fabrication and application of emulsion gels[J]. Journal of Food Safety and Quality,2022,13(4):1036−1043. doi: 10.3969/j.issn.2095-0381.2022.4.spaqzljcjs202204003 DIAO X Q, LI X, SUN W T, et al. Research progress in the fabrication and application of emulsion gels[J]. Journal of Food Safety and Quality, 2022, 13(4): 1036-1043. doi: 10.3969/j.issn.2095-0381.2022.4.spaqzljcjs202204003
[10] POYATO C, ASTIASÁR N I, BARRIUSO B, et al. A new polyunsaturated gelled emulsion as replacer of pork back-fat in burger patties: Effect on lipid composition, oxidative stability and sensory acceptability[J]. LWT-Food Science and Technology,2015,62(2):1069−1075. doi: 10.1016/j.lwt.2015.02.004
[11] 汤洋, 高成成, 张岩, 等. 多糖基颗粒稳定的Pickering 乳液凝胶研究进展[J]. 食品科学,2022,43(3):341−351. [TANG Y, GAO C C, ZHANG Y, et al. A review of literature on Pickering emulsion gels stabilized by polysaccharide-based particles[J]. Food Science,2022,43(3):341−351. doi: 10.7506/spkx1002-6630-20201030-316 TANG Y, GAO C C, ZHANG Y, et al. A review of literature on Pickering emulsion gels stabilized by polysaccharide-based particles[J]. Food Science, 2022, 43(3): 341-351. doi: 10.7506/spkx1002-6630-20201030-316
[12] HU B, WANG S S, LI J, et al. Assembly of bioactive peptide-chitosan nanocomplexes[J]. The Journal of Physical Chemistry B,2011,115(23):7515−7523. doi: 10.1021/jp2013557
[13] CHEN Q, ZHENG J, XU Y, et al. Surface modification improves fabrication of Pickering high internal phase emulsions stabilized by cellulose nanocrystals[J]. Food Hydrocolloids,2017,75:125−130.
[14] RUIZ-CAPILLAS C, HERRERO A M. Development of meat products with healthier lipid content: Vibrational spectroscopy[J]. Foods,2021,10(2):341. doi: 10.3390/foods10020341
[15] HERRERO A M, CARMONA P, JIMÉNEZ-COLMENERO F, et al. Polysaccharide gels as oil bulking agents: Technological and structural propertie[J]. Food Hydrocolloids,2014,36(2):374−381.
[16] PARK D, XIONG Y L, ALDERTON A L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein[J]. Food Chemistry,2007,101(3):1239−1246. doi: 10.1016/j.foodchem.2006.03.028
[17] 朱秀清, 王婵, 孙禹凡, 等. 多糖对大豆分离蛋白乳液及乳液凝胶性质影响[J]. 东北农业大学学报,2019,51(2):45−52. [ZHU X Q, WANG C, SUN Y F, et al. Effect of polysaccharides on soybean protein isolate emulsion and emulsion gel[J]. Journal of Northeast Agricultural University,2019,51(2):45−52. ZHU X Q, WANG C, SUN Y F, et al. Effect of polysaccharides on soybean protein isolate emulsion and emulsion gel[J]. Journal of Northeast Agricultural University, 2019, 51(2): 45-52.
[18] KHALESI H, EMADZADEH B, KADKHODAEE R, et al. Effect of Persian gum on whey protein concentrate cold-set emulsion gel: Structure and rheology study[J]. International Journal of Biological Macromolecules,2019,125:17−26. doi: 10.1016/j.ijbiomac.2018.12.051
[19] ZHANG Q T, TU Z C, XIAO H, et al. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate[J]. Food and Bioproducts Processing,2014,92(1):30−37. doi: 10.1016/j.fbp.2013.07.006
[20] 江连洲, 温家煜, 王禹涵, 等. SPI凝胶颗粒制备及其Pickering高内相乳液特性研究[J]. 农业机械学报,2020,51(12):348−355. [JIANG L Z, WEN J Y, WANG Y H, et al. Fabrication and characterization of high internal phase pickering emulsion manipulated by gel particles of soy protein[J]. Transactions of the Chinese Society of Agricultural Machinery,2020,51(12):348−355. doi: 10.6041/j.issn.1000-1298.2020.12.038 JIANG L Z, WEN J Y, WANG Y H, et al. Fabrication and characterization of high internal phase pickering emulsion manipulated by gel particles of soy protein[J]. Transactions of the Chinese Society of Agricultural Machinery, 2020, 51(12): 348-355. doi: 10.6041/j.issn.1000-1298.2020.12.038
[21] BOUTIN C, GIROUX H J, PAQUIN P, et al. Characterization and acid-induced gelation of butter oil emulsions produced from heated whey protein dispersions[J]. International Dairy Journal,2007,17(6):696−703. doi: 10.1016/j.idairyj.2006.08.009
[22] MAJZNER K, WROBEL T P, FEDOROWICZ A, et al. Secondary structure of proteins analyzed ex vivo in vascular wall in diabetic animals using FT-IR spectroscopy[J]. Analyst,2013,138(24):7400−7410. doi: 10.1039/c3an00455d
[23] ZHAO H, WANG Y, LI W, et al. Effects of oligosaccharides and soy soluble polysaccharide on the rheological and textural properties of calcium sulfate-induced soy protein gels[J]. Food and Bioprocess Technology,2017,10(3):556−567. doi: 10.1007/s11947-016-1826-7
[24] TANG C, YANG M, LIU F, et al. Stirring greatly improves transglutaminase-induced gelation of soy protein-stabilized emulsions[J]. LWT-Food Science and Technology,2013,51(1):120−128. doi: 10.1016/j.lwt.2012.11.004
[25] SIZEMORE S, COPE S, ROY A, et al. Slow internal dynamics and charge expansion in the disordered protein CGRP: A comparison with amylin[J]. Biophysical Journal,2015,109(5):1038−1048. doi: 10.1016/j.bpj.2015.07.023
[26] PIKABEA A, AGUIRRE G, MIRANDA J I, et al. Understanding of nanogels swelling behavior through a deep insight into their morphology[J]. Journal of Polymer Science Part A:Polymer Chemistry,2015,53(17):2017−2025. doi: 10.1002/pola.27653
[27] JIANG Y, ZHU Y Z, LI P, et al. Gliadin/amidated pectin core-shell nanoparticles for stabilization of Pickering emulsion[J]. International Journal of Food Science and Technology,2020,55:3278−3288. doi: 10.1111/ijfs.14590
[28] 敬雪莲, 蔡勇建, 陈碧芬, 等. 基于大豆酶解聚集体制备Pickering乳液凝胶及环境稳定性分析[J]. 食品科学,2022,43(20):7−17. [JING X L, CAI Y J, CHEN B F, et al. Analysis of environmental stability of Pickering emulsion gels prepared with insoluble soy peptide aggregates[J]. Food Science,2022,43(20):7−17. doi: 10.7506/spkx1002-6630-20211123-288 JING X L, CAI Y J, CHEN B F, et al. Analysis of environmental stability of Pickering emulsion gels prepared with insoluble soy peptide aggregates[J]. Food Science, 2022, 43(20): 7-17. doi: 10.7506/spkx1002-6630-20211123-288
[29] 袁华根, 施帅, 奚照寿, 等. 基于MP-KG凝胶体系研究微观结构影响质构特性的调控机制[J]. 食品科学,2022,43(16):129−134. [YUAN H G, SHI S, XI Z S, et al. Insight into the regulatory mechanism of the microstructure of myofibrillar protein-konjac glucomannan gel systems on their textural properties[J]. Food Science,2022,43(16):129−134. doi: 10.7506/spkx1002-6630-20210528-342 YUAN H G, SHI S, XI Z S, et al. Insight into the regulatory mechanism of the microstructure of myofibrillar protein-konjac glucomannan gel systems on their textural properties[J]. Food Science, 2022, 43(16): 129-134. doi: 10.7506/spkx1002-6630-20210528-342
[30] KUHUN K R, GNGELO L F C, CUNHA R L D. Cold-set whey protein flaxseed gum gels induced by mono or divalent salt addition[J]. Food Hydrocolloids,2011,25(5):1302−1310. doi: 10.1016/j.foodhyd.2010.12.005
[31] 任菲. 木薯变性淀粉对乳清蛋白凝胶特性影响[D]. 济南: 齐鲁工业大学, 2017 REN F. Effect of cassava modified starch on the gelling properties of whey protein[D]. Jinan: Qilu University of Technology, 2017.
[32] CARBONARO M, NUCARA A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region[J]. Amino Acids,2010,38(3):679−690. doi: 10.1007/s00726-009-0274-3
[33] ZHOU Y, ZHAO D, FOSTER T J, et al. Konjac glucomannan-induced changes in thiol/disulphide exchange and gluten conformation upon dough mixing[J]. Food Chemistry,2014,150:164−165. doi: 10.1016/j.foodchem.2013.11.001
[34] 潘卓官, 邹怡茜, 陈海强, 等. 温-压结合处理对肌原纤维蛋白结构及凝胶特性的影响研究进展[J/OL]. 食品工业科技: 1−20 [2023-04-06]. DOI:10.13386/j. issn1002-0306.2022060297. PAN Z G, ZOU Y Q, CHEN H Q, et al. Effects of high hydrostatic pressure combined with heat treatment on the structure and gel properties of myofibrillar protein: A review[J/OL]. Science and Technology of Food Industry: 1−20 [2023-04-06]. DOI: 10.13386/j.issn1002-0306.2022060297.
[35] NICOLAI T, CHASSENIEUX C. Heat-induced gelation of casein micelles[J]. Food Hydrocolloids,2021,118(6):106755.
[36] ZHANG H, TAN S M, GAN H M, et al. Investigation of the formation mechanism and β-carotene encapsulation stability of emulsion gels based on egg yolk granules and sodium alginate[J]. Food Chemistry,2022,400(2):134032.
[37] 乔蕾蕾, 杨敏, 秦娟娟, 等. 酸诱导“酪蛋白胶束-海藻酸钠”乳液凝胶性质及其对原花青素的负载性能研究[J/OL]. 食品科学: 1−16 [2023-04-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221229.1924.011.html. QIAO L L, YANG M, QIN J J, et al. Properties of acid-induced “micellar casein-sodium alginate” emulsion gels and their loading capacity for proanthocyanidins[J/OL]. Food Science: 1−16 [2023-04-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221229.1924.011.html.
[38] LIU Q, CHANG X, SHAN Y, et al. Fabrication and characterization of Pickering emulsion gels stabilized by zein/pullulan complex colloidal particles[J]. Journal of the Science of Food and Agriculture,2021,101(9):3630−3643. doi: 10.1002/jsfa.10992
[39] LI S N, ZHANG B, LI C, et al. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network[J]. Food Chemistry,2020,305:125467.
[40] LIN D Q, KELLY A L, MIAO S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene[J]. Food Chemistry,2022,372:131262. doi: 10.1016/j.foodchem.2021.131262
[41] 王旭, 李昕, 许朵霞, 等. 大豆多糖对乳清分离蛋白-乳状液稳定性与流变特性影响[J]. 食品工业科技,2017(19):7−12. [WANG X, LI X, XU D X, et al. Influence of soybean polysaccharide on the physical stability and rheological properties of whey protein isolate emulsion[J]. Science and Technology of Food Industry,2017(19):7−12. WANG X, LI X, XU D X, et al. Influence of soybean polysaccharide on the physical stability and rheological properties of whey protein isolate emulsion[J]. Science and Technology of Food Industry, 2017, (19): 7-12.
[42] TANG C H. Effect of thermal pretreatment of raw soymilk on the gel strength and microstructure of tofu induced by microbial transglutaminase[J]. LWT-Food Science and Technology,2007,40(8):1409.
[43] 孙雅晖, 韩立娟, 苏凌志, 等. κ-卡拉胶对蜂蜡基乳液凝胶微观结构和性质的影响[J/OL]. 食品科学: 1−15 [2023-04-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221207.1626.027.html. SUN Y H, HAN L J, SU L Z, et al. Effect of κ-carrageenan on the microstructure and properties of beeswax-based emulsion gels[J/OL]. Food Science: 1−15 [2023-04-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221207.1626.027.html.









 下载:
下载:
 下载:
下载:
