Research Progress of Signal Amplification Strategies in Immunochromatographic Test Strip
-
摘要: 免疫层析试纸条(Immunochromatographic Test Strip, ICTS)是建立在毛细管层析技术和抗原-抗体特异性反应基础上的一种检测技术,具有成本低、操作简单、不需要专业人员、分析时间短、特异性强和结果肉眼可见等特点,目前被广泛应用于食品安全快速检测中。基于传统球状金纳米材料的ICTS是目前最为常见的方法。但是传统方法仅能实现定性或半定量检测,其低检测灵敏度无法满足现在的检测需求。因此,利用信号放大技术来提高ICTS的灵敏度越来越受到人们的关注。本文总结了ICTS的信号放大策略并提出了未来的发展方向,以期为食品安全快速检测技术的发展提供技术参考。Abstract: Immunochromatographic Test Strip (ICTS), a detection technology based on capillary column chromatography and specific immune interaction between antibody and antigen, is widely utilized in the field of rapid detection of food safety detection due to its cost-effectiveness, easy operation, non-professional personnel, short assay time, high specificity and naked eye visualization. Conventional spherical gold nanoparticles-based ICTS is the most common detection method. However, the conventional ICTS can help achieve simple qualitative or semiquantitative analysis, its low sensitivity is an obstacle to meet the current detection requirements. Therefore, there has been a growing interest focusing on the signal amplification method to improve the sensitivity of ICTS devices. This paper summarizes signal amplification strategies to enhance the sensitivity of ICTS and proposes the future perspectives, which provides technical references for the development of rapid detection methods for food safety.
-
Keywords:
- immunochromatographic /
- test strip /
- signal amplification /
- food safety
-
随着经济全球化和食品国际贸易的发展,食品从生产、加工、包装、运输、储存到消费的各个环节均比以往更容易被食源性致病微生物、农药、兽药、激素和非法添加物等污染。为了保障人民的健康,食品安全的检测技术显得尤为重要。在对食品中有毒有害物质进行检测时,基于高效液相色谱法(high performance liquid chromatography,HPLC)、气相色谱质谱联用法(gas chromatography-mass spectrometry,GC-MS)、液相色谱串联质谱法(liquid chromatography-tandem mass spectrometry,LC-MS)、酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)等仪器分析方法,虽然检测灵敏度较高,结果准确性较好,但是样品前处理比较繁琐,检测时间较长,设备昂贵,需要专业的技术人员操作仪器,因此,不适用于食品安全的现场快速检测[1]。免疫层析试纸条(Immunochromatographic Test Strip,ICTS)结合了层析技术的分离能力和免疫分析方法的特异性,具有操作简单、无需专业技术人员、检测成本较低、检测快速(测定结果可在10~20 min内进行判定)等特点,特别适用于在实验室外对大批量食品样品的快速检测。然而传统的基于球状金纳米材料的ICTS,检测灵敏度相对较低,仅可实现定性和半定量的检测,因此,依赖于ICTS的信号放大技术,通过信号放大技术提高ICTS的检测灵敏度可满足当前对食品安全检测的要求。本文对ICTS的信号放大策略进行综述并提出了未来的发展方向,以期为食品安全快速检测技术的发展提供技术参考。
1. 传统免疫层析试纸条的检测原理
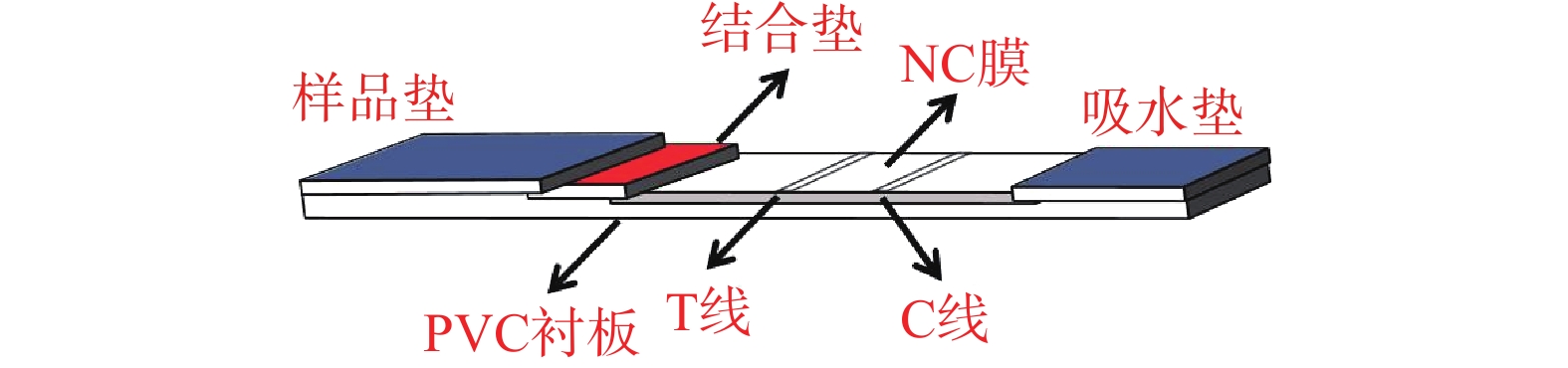
传统的ICTS主要由样品垫、结合垫、硝酸纤维素(nitrocellulose,NC)膜、吸水垫以及PVC衬板五部分组成,其相互堆叠顺序如图1所示。在结合垫上固定有球状金纳米材料标记的抗体,硝酸纤维素膜上划有两条预先固定免疫试剂的检测线(test line,T线)和质控线(control line,C线),T线用于判定检测结果,C线用于判断试纸条的有效性。
传统基于球状金纳米材料的ICTS的检测原理是样品借助层析作用沿试纸条流动,然后与结合垫上的金标抗体和固定于NC膜T线和C线上的免疫试剂相互作用,从而产生红色的条带,用于评估测试结果[2-4]。分为两种模式:双抗体夹心法和竞争抑制法。双抗体夹心法采用一对单克隆抗体,其中一个抗体标记金纳米材料,另外一个抗体喷涂于T线,在有目标物存在的情况下,即可在T线处形成金纳米材料-单克隆抗体1-目标物-单克隆抗体2的夹心结构,该方法主要用于检测大分子目标物(蛋白质、病原菌、病毒等),检测直观性强,灵敏度高,但是需要开发一对抗体和大分子目标物的不同抗原表位结合,无疑增加了检测的成本。竞争法则用于具有单一抗原表位的小分子抗原的检测,如:农兽药残留、激素、毒素等。待检目标小分子物质和喷涂于T线上的抗原竞争地和纳米金标记的抗体相结合。该方法抗体和小分子的亲和性对检测灵敏度的影响很大。两种模式下结合垫、T线和C线固定的免疫试剂及结果判定方法不同,其区别如表1所示。
表 1 夹心免疫层析和竞争性免疫层析的区别Table 1. Difference between sandwich and competitive spherical gold-based immunochromatography assay结合垫 检测线 质控线 结果判定方法 双抗体夹心法 Ab1 Ab2 Ab3 T线越深,目标物越多 竞争抑制法 Ab1 ag Ab3 T线越浅,目标物越多 注:Ab1为金标记的抗目标物抗体;Ab2为抗目标物抗体;Ab3为抗Ab1抗体;ag为抗原。 2. 免疫层析试纸条信号放大方法
2.1 开发本身信号强度高的纳米标记材料
球状金纳米材料是ICTS中最常用的信号标记材料,传统的ICTS通常采用30~40 nm的球状金纳米材料,但是该粒径范围的金纳米材料发光强度不足,且对抗体的捕获率不高(<5%),最终导致试纸条的检测灵敏度不高[5]。100 nm的球状纳米金由于具有较高的摩尔消光系数和光强度,被认为是制作试纸条较为理想的粒径[6]。而当纳米金的粒径过大时,由于存在空间位阻效应和较强的光散射性,会影响试纸条的检测性能[7]。金纳米材料的粒径虽然对ICTS的检测性能有较大影响,但是仅通过优化金纳米材料的粒径并不能满足对目标物质高灵敏的检测需求,因此需要合成本身发光强度更高的新型纳米标记材料用于试纸条的信号放大。表2列出了在ICTS中采用的本身信号强度较高的纳米材料,各类方法相较于传统的基于球状金纳米材料的ICTS的性能提高情况,检测目标物和检测限。
表 2 本身信号强度高的纳米材料Table 2. Nanoparticles with high signal intensity in ICTS2.2 纳米材料聚集法信号放大技术
单个的纳米材料发光强度是有限的,为了增强检测信号强度,研究者开始探索将纳米材料聚集在一起来实现信号增强的目的。第一种使纳米材料聚集的方法是借助中间载体,将纳米材料大量聚集于该载体上或者被包裹在该载体内,进而形成新的信号标记材料,以该材料作为试纸条的信号标签,由于其带有较多的纳米材料,纳米材料的聚集而使得该标签具有更强的发光强度,因此在试纸条的检测过程中可以在测试线上获得更深的条带颜色,由此实现放大检测信号的目的[17]。表3列出了第一种使得纳米材料聚集的方法以实现信号放大目的的ICTS,总结了采用的中间载体、被富集的纳米材料、性能提高倍数、检测目标物及检测限。
表 3 纳米材料聚集法实现信号放大的ICTSTable 3. Nanoparticles aggregations method for signal amplification in ICTS被富集的纳米材料 中间载体 性能提高倍数
(vs基于球状金纳米材料
的ICTS,特殊说明除外)检测目标物 检测限 参考文献 金纳米材料 PAMAM 50倍 双酚A 10 ng/mL [18] 金纳米材料 细菌 20倍 盐酸克伦特罗 0.1 ng/mL [19] 金纳米材料 酵母菌/乳酸菌 8倍 赭曲霉毒素A 0.1 ng/mL [20] CdSe/ZnS量子点 二氧化硅@聚乙烯亚胺 20倍(vs基于量子点的ICTS) 鼠伤寒沙门氏菌 5×102 CFU/mL [21] 金纳米材料 聚多巴胺 10倍 玉米赤霉烯酮 7.4 pg/mL [22] 金纳米材料 氮化碳 3倍 17 β-雌二醇 0.5 ng/mL [23] CdSSe/ZnS量子点 Fe3O4@SiO2 4倍 盐酸克伦特罗 0.16 ng/mL [24] 金纳米材料 锆金属有机框架结构 — 呋喃唑酮代谢物 0.6 ng/mL [25] 量子点 二氧化硅 200倍 鼠伤寒沙门氏菌 50 CFU/mL [26] 大肠杆菌O157:H7 50 CFU/mL 铕纳米材料 聚苯乙烯 100倍 鼠伤寒沙门氏菌 103 CFU/mL [27] 金纳米材料 聚合物 — 赭曲霉毒素A 0.094 ng/mL [28] 氧化铁纳米材料 金纳米材料 聚合物 提高(vs基于荧光纳米材料的ICTS) 黄曲霉毒素B1 3 pg/mL [29] 氧化铁纳米材料 CdSSe/ZnS量子点 Fe3O4@SiO2 — 大肠杆菌O157:H7 2.39×102 CFU/mL [30] CdS0.75Se0.25量子点 金黄色葡萄球菌 17倍 玉米赤霉烯酮 0.0058 ng/mL [31] 钌 罗丹明B 脂质体 5个数量级 沙门氏菌 102 CFU/mL [32] 金纳米材料 二氧化锰纳米花 58倍 脱氧雪腐镰刀菌烯醇 0.013 ng/mL [33] 金纳米材料 聚苯乙烯乳胶微球 64倍 流感病毒 0.016 HAU [34] 第二种使纳米材料聚集的方法是双层法,即采用一对可特异性结合的物质分别结合纳米材料,目前利用的该类物质主要为抗体-抗抗体、生物素和链霉亲和素(streptavidin,Sa)以及牛血清白蛋白(bovine serum albumin,BSA)-抗BSA的抗体,在免疫层析的过程中,利用这些物质之间的相互作用实现纳米材料的聚集进而增强检测信号的发光强度。Chen等[35]将金纳米材料分别标记抗体和抗抗体,免疫层析的进行即可在试纸条测试上形成纳米金-抗体-抗抗体-纳米金复合物进而实现了金纳米材料的聚集,用该方法检测大肠杆菌O157:H7,对其检测限为1.14×103 CFU/mL。Yan等[36]则利用抗体和抗抗体之间的相互作用实现了磁性纳米材料的聚集,对呋喃唑酮代谢物的检测限为0.044 ng/mL,和传统方法相比,灵敏度提高了10倍。Fang等[37]将金纳米材料分别标记链霉亲和素和生物素化的单克隆抗体,进而形成纳米金-Sa-生物素-抗体-纳米金复合物,该方法实现对农药吡虫啉的检测,灵敏度为传统方法的160倍。用于试纸条的纳米信号标记材料通常会采用BSA来封闭非特异性的结合位点,Zhong等[38]将金纳米材料分别标记单克隆抗体和抗BSA的抗体,进而形成纳米金-抗BSA抗体-BSA-纳米金-单克隆抗体复合物,该方法对食品中的三聚氰胺的检测限为1.4 ng/mL,检测灵敏度和传统方法相比提高了25倍。双层法虽然提高了ICTS的检测灵敏度,但是繁琐的双标记过程增加了试纸条的制备时间,影响试纸条的大规模生产,同时会存在一定的空间位阻效应影响待检目标物和抗体之间的结合效率,影响检测结果的重复性。
2.3 应用增强试剂信号放大技术
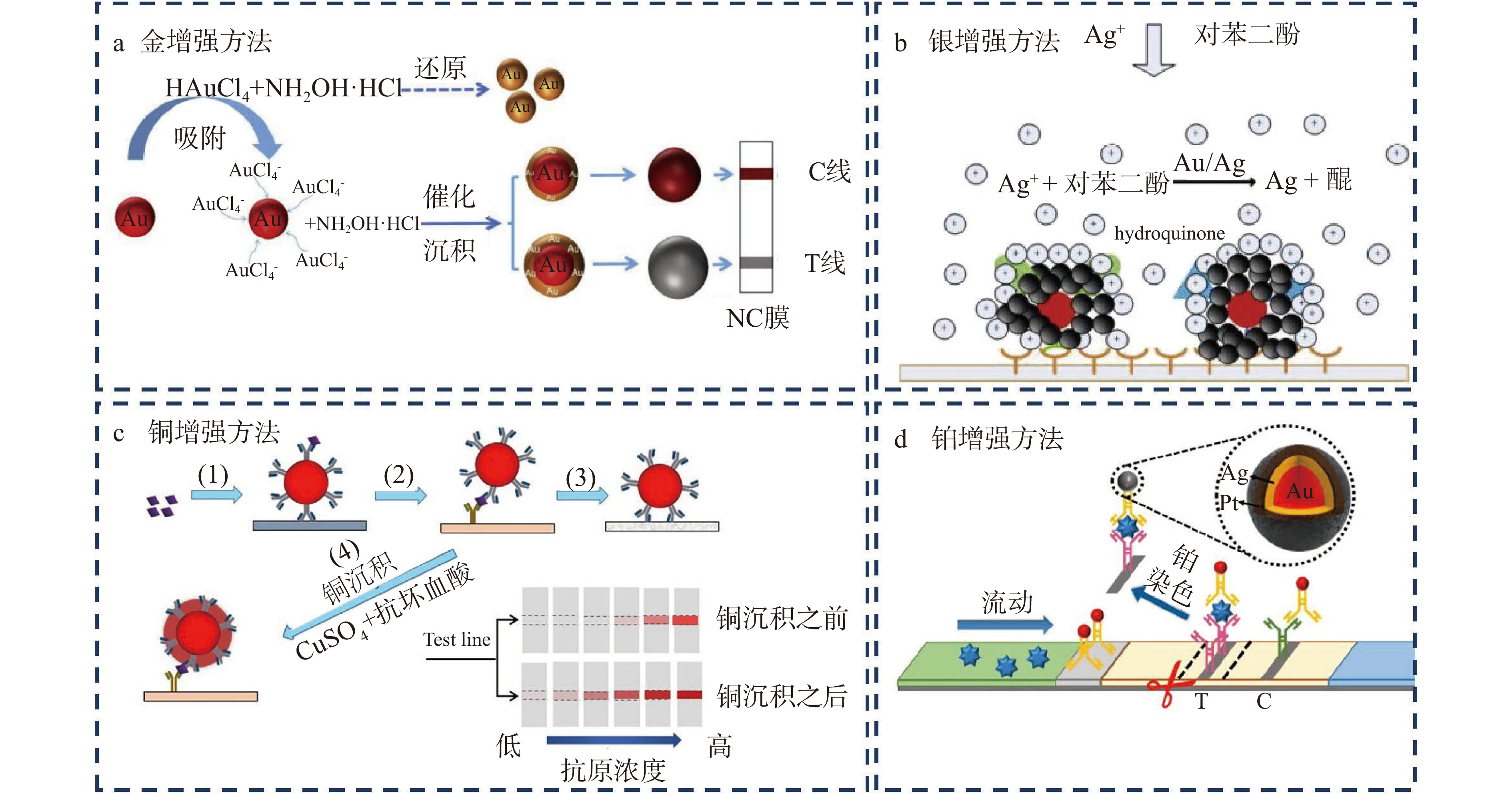
在ICTS中通过应用增强试剂之间的相互作用,反应会形成新的金属而在试纸条的测试线上沉积,金属的沉积加深了测试线的颜色,进而提高了检测的灵敏度。目前应用增强试剂放大信号的方法主要有“金增强方法”、“银增强方法”、“铂增强方法”以及“铜增强方法”。被称为“金增强方法”的信号放大策略是利用金增强试剂氯金酸(HAuCl4)和还原剂盐酸羟胺(NH2OH·HCl)之间的反应,在试纸条原始的纳米金表面形成新的一层金纳米颗粒,从而增大金纳米材料的尺寸而实现信号放大的目的(图2a)。与直接合成大粒径金纳米材料信号放大的方法相比,该方法是在常规免疫层析结束后再引入增强试剂继续增大纳米材料的粒径。因此材料粒径增大并不会影响抗原抗体的结合效率,不存在空间位阻效应影响反应的灵敏度。Bu等[39]利用该策略检测食品中的肠炎沙门氏菌,检测信号增强了100倍。银增强方法是在试纸条金纳米材料的催化下,还原剂对苯二酚将银盐溶液中的银离子还原为银单质,银单质沉积于原先的金纳米材料上,形成银-金纳米复合物,该复合物在试纸条上形成黑色的检测条带(图2b)。与原始的红色条带相比,黑色条带颜色较深,且和试纸条白色的背景对比更加明显[40]。Yu等[41]利用该方法检测伏马菌素B1和脱氧雪腐镰刀菌烯醇,对其检测限分别为2和40 ng/mL,检测限和传统方法相比至少降低了两倍。Anfossi等[42]利用银增强方法检测食品中的赭曲霉毒素A,灵敏度和传统方法相比提高了10倍。虽然上述方法可提高试纸条的检测灵敏度,但是添加银增强试剂需要额外的二次操作,因此会延长试纸条的分析时间,为了解决这一问题,Kim等[43]将银增强试剂和水溶性的核壳纳米纤维相结合,并将该纳米纤维置于试纸条检测线之前,随着免疫层析过程的进行,银增强试剂可从纤维中释放,从而实现一步信号放大的目的,该方法对目标物的检测灵敏度提高了10倍,但是并不影响分析时间。铜增强方法是利用抗坏血酸将Cu2+还原为金属铜单质(图2c),铜单质在测试线上的沉积增强了测试线的颜色[44]。Zhou等[45]利用该方法对牛奶中的大肠杆菌O157:H7进行检测,检测限可达到6 CFU/mL。铂增强方法是利用对苯二酚将银离子还原为金属银单质,然后利用抗坏血酸将氯铂酸(H2PtCl6)中的Pt4+还原为金属铂而沉积到测试线上(图2d),测试线颜色加深使得检测灵敏度提高,该方法对肌红蛋白的检测限为5.47 ng/mL[46]。
应用增强试剂的信号放大技术虽然提高了试纸条的检测灵敏度,但是添加增强试剂通常是在常规免疫层析之后进行的二次操作,会增加试纸条的分析时间,影响检测效率。因此一步法实现信号放大的技术是未来研究的重点方向。
2.4 纳米材料修饰酶信号放大技术
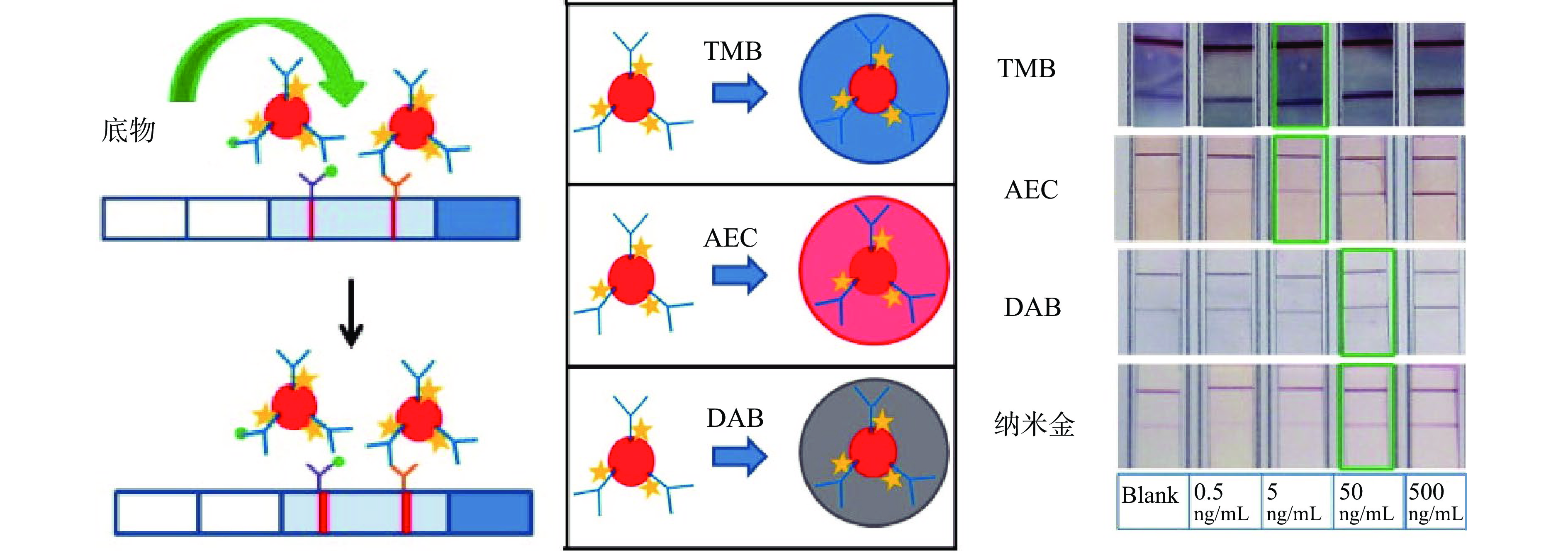
该方法是将纳米材料同时标记蛋白酶和单克隆抗体,利用蛋白酶催化底物的高效性和产物的显色性,使得试纸条检测线颜色加深而实现信号放大的目的[47-49]。目前,辣根过氧化物酶(horseradish peroxidase,HRP)在ICTS信号放大技术中应用较为广泛,其催化效率高,一个酶分子可在1 s内催化产生103个颜色产物[50]。Parolo等[51]开发了基于HRP催化底物的信号放大ICTS,同时研究了三种不同类型的HRP催化底物3, 3’, 5, 5’-四甲基联苯胺(tetramethylbenzidine,TMB)、3, 3’-二氨基联苯胺盐酸盐(diaminobenzidine tetrahydrochloride,DAB)和3-氨基-9-乙基咔唑(3-amino-9-ethyl-carbazole,AEC)对试纸条检测性能的影响(图3),结果表明TMB是HRP催化底物信号放大技术中理想的物质选择。Panferov等[52]则利用碱性磷酸酶实现试纸条的信号放大,用该方法检测马铃薯X病毒,对其检测限为0.3 ng/mL,检测限比常规试纸条降低了27倍。
该方法虽然对ICTS的性能提升效果明显,信号放大过程简单(室温、水溶液、5 min反应时间),但是蛋白酶相对较差的热稳定性和化学稳定性是一个潜在的问题,同时酶分子会和抗体竞争地结合在纳米材料的表面,导致抗体在纳米材料上的结合量减少,酶的空间位阻效应还可能会影响抗体与分析物结合的效率。酶的结合量以及最终信号放大的幅度会受到纳米材料有限表面积的限制[53]。
2.5 利用纳米催化材料信号放大技术
纳米催化材料,也称为纳米酶,是一种基于纳米材料的人工酶,其具有和酶相似的催化活性,催化性能受外界环境条件的影响较小,化学性质稳定,易于表面修饰,同时比蛋白酶的生产成本低[54]。利用纳米酶催化底物产生的颜色而增强测试线的信号也可实现ICTS的信号放大。Loynachan等[55]在15 nm的纳米金颗粒上生长铂,进而合成多孔的核壳纳米催化剂,以该材料作为试纸条的信号标签(图4a),对目标物质的检测限可降低100倍。Gao等[56]在金纳米材料表面覆盖铂层,以形成的纳米金@铂作为信号标记材料,由于铂的高效过氧化物模拟酶活性,其催化底物显色使得试纸条的检测灵敏度提高了两个数量级,如图4b所示。
近年来应用于ICTS中的纳米催化材料以及使用催化材料之后试纸条的性能提高情况如表4所示。
表 4 在ICTS中为实现信号放大的目的使用的纳米催化材料Table 4. Catalytic nanomaterials used in ICTS for signal amplification purpose2.6 富集样品中检测目标物信号放大技术
近年来富集样品中检测目标物的方法主要采用磁性分离技术,因为磁性纳米材料合成成本低廉、结构易于控制、具有良好的生物相容性,特别是可以由一块简单的磁铁进行富集操作,使所有的分离过程都在一个离心管中完成,无需耗时的离心过程。在对检测目标物进行富集时,将抗体偶联的磁性纳米材料添加到样品基质中,孵育一定时间后,在样品基质中会形成抗原-抗体-磁性纳米材料复合物,然后使用外部磁场的作用提取该复合物,再将分离物重悬到溶液中(磷酸盐缓冲溶液或去离子水),使用该重悬液进行ICTS快速检测分析。因此磁性分离技术除了可以富集检测目标物以外,还可以将目标物从复杂的样品基质中分离出来,进而可消除试纸条检测过程中样品基质的干扰,提高检测的灵敏度[64-67]。Zhang等[27]采用免疫磁分离技术对待检样品进行预处理,富集样品中的待检目标物,同时消除样品基质的影响(图5a)。对鼠伤寒沙门氏菌的检测限为103 CFU/mL。Guo等[29]采用磁分离技术对酱油中的黄曲霉毒素进行分离和富集,同时消除了酱油黑色的样品基质对ICTS检测过程中的干扰(图5b),也可提高检测的灵敏度。
2.7 降低层析速度法信号放大技术
在ICTS中,试剂层析的速度相对较快,导致免疫试剂之间的结合时间较短,通过降低液体的流动速度可以提高免疫试剂之间的结合效率,使得试纸条检测线上结合的信号标签增多,最终起到ICTS信号放大的目的。Tsai等[68]在测试区域和结合垫之间额外增加一个“叠加垫”(图6),这种设计有助于将抗体和抗原积累在“叠加垫”上进行反应,从而减慢免疫试剂的流动速度,延长抗原/抗体的相互作用时间,提高试验的检测灵敏度。与传统的ICTS测试相比,该方法可以提高2倍的检测信号。
近年来通过降低层析速度提高ICTS灵敏度的方法如表5所示,同时列出了和传统的不降低层析速度的方法相比试纸条的性能提高情况。
表 5 降低免疫层析速度实现信号放大的ICTSTable 5. Reduce the immunochromatographic speed for signal amplification in ICTS2.8 非比色法信号放大技术
在传统的ICTS中,作为信号标签的纳米材料,其光学特性通常作为信号输出的基础,检测结果采用比色的方法,导致纳米材料所具有的出色的光热传感能力和表面增强拉曼(Surface-enhanced Raman Spectroscopy,SERS)信号的能力被忽视。研究表明,利用纳米材料的热信号和表面增强拉曼信号对目标物质进行分析,可提高ICTS的灵敏度[79-85]。
金纳米材料在光刺激下通过表面等离子体共振(surface plasmon resonance,SPR)产生热,特别是在吸收532 nm的光时。Zhang等[86]建立了一种基于金纳米材料光热效应的快速定量试纸条检测方法,该检测方法利用激光笔和温度传感器用于记录温度信号(图7a),光热方法对盐酸克伦特罗和鼠伤寒沙门氏菌的检测限降低了一个数量级。Zhang等[33]则建立了基于纳米金功能化花状二氧化锰纳米材料光热效应的ICTS,该方法对脱氧雪腐镰刀菌烯醇的检测限为0.013 ng/mL,灵敏度和传统的基于金纳米材料的试纸条相比提高了58倍(图7b),同时该方法消除了肉眼在低目标物浓度下无法区分测试条差异的缺点。
SERS是一种超灵敏的振动光谱技术,具有抗光漂白、响应时间短、波段分辨率高、信息丰富等优点。基于SERS的免疫层析方法不仅具有SERS的高灵敏度和光谱分辨的能力,而且具有免疫层析技术的特异性和便捷性。一个典型的SERS信号标签由三部分组成:金或者银纳米颗粒作为拉曼增强的基底、吸附在材料上的信号分子用于产生特征拉曼信号、特异性抗体用于识别目标分析物[87-89]。Lin等[90]通过在金纳米星表面吸附4-氨基苯硫酚分子,以其作为试纸条的信号标签,通过采集试纸条的拉曼信号,对双酚A的检测限为0.073 ng/mL(图8a),灵敏度是基于比色方法的20倍。Su等[91]开发了纳米金为核,金纳米星为壳的核壳纳米材料,拉曼信号分子5,5'-二巯基(2-硝基苯甲酸)位于核壳的中间,以形成的夹心结构的纳米材料作为ICTS拉曼信号标签(图8b),对盐酸克伦特罗进行检测,对其检测限为0.05 ng/mL,灵敏度比传统的基于金纳米材料的试纸条提高了200倍。
3. 结语
ICTS经过多年的发展在食品安全快速检测领域已经占据重要地位,传统的基于球状金纳米材料的ICTS检测灵敏度低,不能满足食品安全高灵敏检测的需求。本文总结了近年来ICTS信号放大技术,尽管使得ICTS的检测性能有了极大的提升,但在实际应用过程中仍然存在很多问题。目前存在的主要问题及未来的发展方向简述如下:a.新型纳米标记材料的合成过程相对复杂,耗时耗力,有些材料还需要比较严苛的合成条件,同时存在材料稳定性较差的问题,因此,开发合成简单快速、性质稳定的纳米材料仍然是目前研究的一大方向;b.基于增强试剂的信号放大方法需要额外的二次操作以滴加试剂,无疑增加了试纸条的分析时间,因此,开发一步信号放大的方法可极大地提升试纸条的检测性能;c.基于光热和拉曼信号的试纸条,需要相应的传感器,增加了试纸条检测的成本,开发便携廉价的传感器是未来发展的趋势;d.在试纸条上设置障碍降低免疫试剂的流动速度,虽然可使试剂反应时间增长,提高检测的灵敏度,但是障碍物在试纸条上的设置比较复杂,使试纸条难以实现大规模生产,因此,亟需开发简单易行的方法来降低流体层析的速度,使得试纸条的生产简单化。
-
表 1 夹心免疫层析和竞争性免疫层析的区别
Table 1 Difference between sandwich and competitive spherical gold-based immunochromatography assay
结合垫 检测线 质控线 结果判定方法 双抗体夹心法 Ab1 Ab2 Ab3 T线越深,目标物越多 竞争抑制法 Ab1 ag Ab3 T线越浅,目标物越多 注:Ab1为金标记的抗目标物抗体;Ab2为抗目标物抗体;Ab3为抗Ab1抗体;ag为抗原。 表 2 本身信号强度高的纳米材料
Table 2 Nanoparticles with high signal intensity in ICTS
表 3 纳米材料聚集法实现信号放大的ICTS
Table 3 Nanoparticles aggregations method for signal amplification in ICTS
被富集的纳米材料 中间载体 性能提高倍数
(vs基于球状金纳米材料
的ICTS,特殊说明除外)检测目标物 检测限 参考文献 金纳米材料 PAMAM 50倍 双酚A 10 ng/mL [18] 金纳米材料 细菌 20倍 盐酸克伦特罗 0.1 ng/mL [19] 金纳米材料 酵母菌/乳酸菌 8倍 赭曲霉毒素A 0.1 ng/mL [20] CdSe/ZnS量子点 二氧化硅@聚乙烯亚胺 20倍(vs基于量子点的ICTS) 鼠伤寒沙门氏菌 5×102 CFU/mL [21] 金纳米材料 聚多巴胺 10倍 玉米赤霉烯酮 7.4 pg/mL [22] 金纳米材料 氮化碳 3倍 17 β-雌二醇 0.5 ng/mL [23] CdSSe/ZnS量子点 Fe3O4@SiO2 4倍 盐酸克伦特罗 0.16 ng/mL [24] 金纳米材料 锆金属有机框架结构 — 呋喃唑酮代谢物 0.6 ng/mL [25] 量子点 二氧化硅 200倍 鼠伤寒沙门氏菌 50 CFU/mL [26] 大肠杆菌O157:H7 50 CFU/mL 铕纳米材料 聚苯乙烯 100倍 鼠伤寒沙门氏菌 103 CFU/mL [27] 金纳米材料 聚合物 — 赭曲霉毒素A 0.094 ng/mL [28] 氧化铁纳米材料 金纳米材料 聚合物 提高(vs基于荧光纳米材料的ICTS) 黄曲霉毒素B1 3 pg/mL [29] 氧化铁纳米材料 CdSSe/ZnS量子点 Fe3O4@SiO2 — 大肠杆菌O157:H7 2.39×102 CFU/mL [30] CdS0.75Se0.25量子点 金黄色葡萄球菌 17倍 玉米赤霉烯酮 0.0058 ng/mL [31] 钌 罗丹明B 脂质体 5个数量级 沙门氏菌 102 CFU/mL [32] 金纳米材料 二氧化锰纳米花 58倍 脱氧雪腐镰刀菌烯醇 0.013 ng/mL [33] 金纳米材料 聚苯乙烯乳胶微球 64倍 流感病毒 0.016 HAU [34] 表 4 在ICTS中为实现信号放大的目的使用的纳米催化材料
Table 4 Catalytic nanomaterials used in ICTS for signal amplification purpose
表 5 降低免疫层析速度实现信号放大的ICTS
Table 5 Reduce the immunochromatographic speed for signal amplification in ICTS
-
[1] ZHANG Mengyue, YAN Lingzhi, HUANG Qiong, et al. Highly sensitive simultaneous detection of major ochratoxins by an immunochromatographic assay[J]. Food Control,2018,84:215−220.
[2] ZHANG Daohong, LI Peiwu, YANG Yang, et al. A high selective immunochromatographic assay for rapid detection of aflatoxin B1[J]. Talanta,2011,85(1):736−742. doi: 10.1016/j.talanta.2011.04.061
[3] ZHANG Daohong, LI Peiwu, ZHANG Qi, et al. A naked-eye based strategy for semiquantitative immunochromatographic assay[J]. Analytica Chimica Acta,2012,740:74−79. doi: 10.1016/j.aca.2012.06.015
[4] ZHANG Daohong, LI Peiwu, ZHANG Qi, et al. Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts[J]. Biosensors and Bioelectronics,2011,26(6):2877−2882. doi: 10.1016/j.bios.2010.11.031
[5] ZHAN Li, GUO Shuangzhuang, SONG Fayi, et al. The role of nanoparticle design in determining analytical performance of lateral flow immunoassays[J]. Nano Letters,2017,17(12):7207−7212. doi: 10.1021/acs.nanolett.7b02302
[6] LI Juan, DUAN Hong, XU Peng, et al. Effect of different-sized spherical gold nanoparticles grown layer by layer on the sensitivity of an immunochromatographic assay[J]. RSC Advances,2016,6(31):26178−26185. doi: 10.1039/C6RA03695C
[7] LIU Linyang, YANG Danting, LIU Guozhen. Signal amplification strategies for paper-based analytical devices[J]. Biosensors & Bioelectronics,2019,136:60−75.
[8] JI Yanwei, REN Meiling, LI Yanping, et al. Detection of aflatoxin B1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers[J]. Talanta,2015,142:206−212. doi: 10.1016/j.talanta.2015.04.048
[9] ZHAO Bingxin, HUANG Qiong, DOU Leina, et al. Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol[J]. Sensors and Actuators B: Chemical,2018,275:223−229. doi: 10.1016/j.snb.2018.08.029
[10] YAO Li, TENG Jun, ZHU Mengya, et al. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions[J]. Biosensors & Bioelectronics,2016,85:331−336.
[11] BU Tong, JIA Pei, SUN Xinyu, et al. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples[J]. Sensors and Actuators B: Chemical,2020,320:128440. doi: 10.1016/j.snb.2020.128440
[12] SU Lihong, WANG Lulu, YAO Xiaolin, et al. Small size nanoparticles-Co3O4 based lateral flow immunoassay biosensor for highly sensitive and rapid detection of furazolidone[J]. Talanta,2020,211:120729. doi: 10.1016/j.talanta.2020.120729
[13] SHI Lei, WU Feng, WEN Yiming, et al. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay[J]. Analytical and Bioanalytical Chemistry,2015,407(2):529−535. doi: 10.1007/s00216-014-8276-8
[14] WANG Peilong, WANG Ruiguo, ZHANG Wei, et al. Novel fabrication of immunochromatographic assay based on up conversion phosphors for sensitive detection of clenbuterol[J]. Biosensors & Bioelectronics,2016,77:866−870.
[15] CHEN Yiqiang, CHEN Qian, HAN Miaomiao, et al. Near-infrared fluorescence-based multiplex lateral flow immunoassay for the simultaneous detection of four antibiotic residue families in milk[J]. Biosensors & Bioelectronics,2016,79:430−434.
[16] WANG Jingyun, ZHANG Lei, HUANG Youju, et al. Hollow Au-Ag nanoparticles labeled immunochromatography strip for highly sensitive detection of clenbuterol[J]. Scientific Reports,2017(7):41419.
[17] XU Hui, CHEN Jiao, BIRRENKOTT J, et al. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins[J]. Analytical Chemistry,2014,86(15):7351−7359. doi: 10.1021/ac502249f
[18] PENG Xiayu, KANG Lichao, PANG Fangqin, et al. A signal-enhanced lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A[J]. Food and Agricultural Immunology,2017,29(1):216−227.
[19] HUANG Qiong, BU Tong, ZHANG Wentao, et al. An improved clenbuterol detection by immunochromatographic assay with bacteria@Au composite as signal amplifier[J]. Food Chemistry,2018,262:48−55. doi: 10.1016/j.foodchem.2018.04.085
[20] BU Tong, ZHANG Meng, SUN Xinyu, et al. Gold nanoparticles-functionalized microorganisms assisted construction of immunobiosensor for sensitive detection of ochratoxin A in food samples[J]. Sensors and Actuators B: Chemical,2019,299:126969. doi: 10.1016/j.snb.2019.126969
[21] ZHANG Bo, YANG Xingsheng, LIU Xiaoxian, et al. Polyethyleneimine-interlayered silica-core quantum dot-shell nanocomposites for sensitive detection of Salmonella typhimurium via a lateral flow immunoassay[J]. RSC Advances,2020,10(5):2483−2489. doi: 10.1039/C9RA09252H
[22] XU Shaolan, ZHAGN Ganggang, FANG Bolong, et al. Lateral flow immunoassay based on polydopamine-coated gold nanoparticles for the sensitive detection of zearalenone in maize[J]. ACS Applied Materials & Interfaces,2019,11(34):31283−31290.
[23] YAO Xiaolin, WANG Zonghan, ZHAO Man, et al. Graphite-like carbon nitride-laden gold nanoparticles as signal amplification label for highly sensitive lateral flow immunoassay of 17 beta-estradiol[J]. Food Chemistry,2021,347:129001. doi: 10.1016/j.foodchem.2021.129001
[24] HUANG Zhen, XIONG Zhijuan, CHEN Yuan, et al. Sensitive and matrix-tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine[J]. Journal of Agricultural and Food Chemistry,2019,67(10):3028−3036. doi: 10.1021/acs.jafc.8b06449
[25] YIN Xuechi, DOU Leina, YAO Xiaolin, et al. Controllable assembly metal-organic frameworks and gold nanoparticles composites for sensitive immunochromatographic assay[J]. Food Chemistry,2022,367:130737. doi: 10.1016/j.foodchem.2021.130737
[26] ZHENG Shuai, YANG Xingsheng, ZHANG Bo, et al. Sensitive detection of Escherichia coli O157:H7 and Salmonella typhimurium in food samples using two-channel fluorescence lateral flow assay with liquid Si@quantum dot[J]. Food Chemistry,2021,363:130400. doi: 10.1016/j.foodchem.2021.130400
[27] ZHANG Yunyue, REN Fazheng, WANG Guoxin, et al. Rapid and sensitive pathogen detection platform based on a lanthanide-labeled immunochromatographic strip test combined with immunomagnetic separation[J]. Sensors and Actuators B: Chemical,2021,329:129273. doi: 10.1016/j.snb.2020.129273
[28] HAO Liangwen, CHEN Jing, CHEN Xirui, et al. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice[J]. Food Chemistry,2021,336:127710.
[29] GUO Liang, SHAO Yanna, DUAN Hong, et al. Magnetic quantum dot nanobead-based fluorescent immunochromatographic assay for the highly sensitive detection of aflatoxin B1 in dark soy sauce[J]. Analytical Chemistry,2019,91(7):4727−4734. doi: 10.1021/acs.analchem.9b00223
[30] HUANG Zhen, PENG Juan, HAN Jiaojiao, et al. A novel method based on fluorescent magnetic nanobeads for rapid detection of Escherichia coli O157:H7[J]. Food Chemistry,2019,276:333−341. doi: 10.1016/j.foodchem.2018.09.164
[31] BU Tong, BAI Feier, ZHAO Shuang, et al. Multifunctional bacteria-derived tags for advancing immunoassay analytical performance with dual-channel switching and antibodies bioactivity sustaining[J]. Biosensors & Bioelectronics,2021,192:113538.
[32] SHUKLA S, LEEM H, KIM M, et al. Development of a liposome-based immunochromatographic strip assay for the detection of Salmonella[J]. Anal Bioanal Chem,2011,401(8):2581−2590. doi: 10.1007/s00216-011-5327-2
[33] ZHANG Meng, BU Tong, BAI Feier, et al. Gold nanoparticles-functionalized three-dimensional flower-like manganese dioxide: A high-sensitivity thermal analysis immunochromatographic sensor[J]. Food Chemistry,2021,341:128231. doi: 10.1016/j.foodchem.2020.128231
[34] LIU Xiao, YANG Jifei, LI Qingmei, et al. A strip test for the optical determination of influenza virus H3 subtype using gold nanoparticle coated polystyrene latex microspheres[J]. Microchim Acta,2020,187(5):306. doi: 10.1007/s00604-020-04255-1
[35] CHEN Minghui, YU Zhibiao, LIU Daofeng, et al. Dual gold nanoparticle lateflow immunoassay for sensitive detection of Escherichia coli O157:H7[J]. Analytica Chimica Acta,2015,876:71−76. doi: 10.1016/j.aca.2015.03.023
[36] YAN Lingzhi, DOU Leina, BU Tong, et al. Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe[J]. Food Chemistry,2018,261:131−138. doi: 10.1016/j.foodchem.2018.04.016
[37] FANG Qingkui, WANG Limin, CHENG Qi, et al. A bare-eye based one-step signal amplified semiquantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples[J]. Analytica Chimica Acta,2015,881:82−89. doi: 10.1016/j.aca.2015.04.047
[38] ZHONG Youhao, CHEN Yinji, YAO Li, et al. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening[J]. Microchimica Acta,2016,183(6):1989−1994. doi: 10.1007/s00604-016-1812-9
[39] BU Tong, HUANG Qiong, YAN Lingzhi, et al. Ultra technically-simple and sensitive detection for Salmonella enteritidis by immunochromatographic assay based on gold growth[J]. Food Control,2018,84:536−543. doi: 10.1016/j.foodcont.2017.08.036
[40] PANFEROV V G, SAFENKOVA I V, VARITSEV Y A, et al. Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of Ralstonia solanacearum in potato tubers[J]. Talanta,2016,152:521−530. doi: 10.1016/j.talanta.2016.02.050
[41] YU Qing, LI Heng, LI Chenglong, et al. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol[J]. Food Control,2015,54:347−352. doi: 10.1016/j.foodcont.2015.02.019
[42] ANFOSSI L, DI NARDO F, GIOVANNOLI C, et al. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement[J]. Anal Bioanal Chem,2013,405(30):9859−9867. doi: 10.1007/s00216-013-7428-6
[43] KIM W, LEE S, JEON S. Enhanced sensitivity of lateral flow immunoassays by using water-soluble nanofibers and silver-enhancement reactions[J]. Sensors and Actuators B: Chemical,2018,273:1323−1327. doi: 10.1016/j.snb.2018.07.045
[44] TIAN Meiling, LEI Lingli, XIE Wenyue, et al. Copper deposition-induced efficient signal amplification for ultrasensitive lateral flow immunoassay[J]. Sensors and Actuators B: Chemical,2019,282:96−103. doi: 10.1016/j.snb.2018.11.028
[45] ZHOU Yaofeng, CHEN Yuan, LIU Yang, et al. Controlled copper in situ growth-amplified lateral flow sensors for sensitive, reliable, and field-deployable infectious disease diagnostics[J]. Biosensors & Bioelectronics,2021,171:112753.
[46] HUANG Di, LIN Bingqian, SONG Yanling, et al. Staining traditional colloidal gold test strips with Pt nanoshell enables quantitative point-of-care testing with simple and portable pressure meter readout[J]. Acs Applied Materials & Interfaces,2019,11(2):1800−1806.
[47] LI Y S, ZHOU Y, MENG X Y, et al. Enzyme-antibody dual labeled gold nanoparticles probe for ultrasensitive detection of kappa-casein in bovine milk samples[J]. Biosensors & Bioelectronics,2014,61:241−244.
[48] HENDRICKSON, ZVEREVA E A, ZHERDEV A V, et al. Ultrasensitive lateral flow immunoassay of phycotoxin microcystin-LR in seafood based on magnetic particles and peroxidase signal amplification[J]. Food Control,2022,133:108655. doi: 10.1016/j.foodcont.2021.108655
[49] GAO Xuefei, XU Liping, WU Tingting, et al. An enzyme-amplified lateral flow strip biosensor for visual detection of microRNA-224[J]. Talanta,2016,146:648−654. doi: 10.1016/j.talanta.2015.06.060
[50] GAO Lizeng, ZHUANG Jie, NIE Leng, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles[J]. Nature Nanotechnology,2007,2(9):577−583. doi: 10.1038/nnano.2007.260
[51] PAROLO C, DE LA ESCOSURA-MUNIZ A, MERKOCI A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes[J]. Biosensors and Bioelectronics,2013,40(1):412−416. doi: 10.1016/j.bios.2012.06.049
[52] PANFEROV V G, SAFENKOVA I V, VARITSEV Y A, et al. Enhancement of lateral flow immunoassay by alkaline phosphatase: A simple and highly sensitive test for potato virus X[J]. Microchimica Acta,2017,185(1):25.
[53] YE Haihang, XIA Xiaohu. Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques[J]. Journal of Materials Chemistry B,2018,6(44):7102−7111. doi: 10.1039/C8TB01603H
[54] JIANG Dawei, NI Dalong, ROSENKRANS Z T, et al. Nanozyme: New horizons for responsive biomedical applications[J]. Chem Soc Rev,2019,48(14):3683−3704. doi: 10.1039/C8CS00718G
[55] LOYNACHAN C N, THOMAS M R, GRAY E R, et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range[J]. ACS Nano,2018,12(1):279−288. doi: 10.1021/acsnano.7b06229
[56] GAO Zhuangqiang, YE Haihang, TANG Dianyong, et al. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics[J]. Nano Letters,2017,17(9):5572−5579. doi: 10.1021/acs.nanolett.7b02385
[57] ZHAGN Jing, TANG Lemin, YU Qingcai, et al. Gold-platinum nanoflowers as colored and catalytic labels for ultrasensitive lateral flow MicroRNA-21 assay[J]. Sensors and Actuators B: Chemical,2021,344:130325. doi: 10.1016/j.snb.2021.130325
[58] JIANG Tao, SONG Yang, DU Dan, et al. Detection of p53 protein based on mesoporous Pt-Pd nanoparticles with enhanced peroxidase-like catalysis[J]. ACS Sensors,2016,1(6):717−724. doi: 10.1021/acssensors.6b00019
[59] CHENG Nan, SHI Qiurong, ZHU Chengzhou, et al. Pt-Ni(OH)2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides[J]. Biosensors & Bioelectronics,2019,142:111498.
[60] LIN Shan, ZHENG Danmin, LI Ailing, et al. Black oxidized 3, 3', 5, 5'-tetramethylbenzidine nanowires (oxTMB NWs) for enhancing Pt nanoparticle-based strip immunosensing[J]. Analytical and Bioanalytical Chemistry,2019,411(18):4063−4071. doi: 10.1007/s00216-019-01745-x
[61] LIU Sijie, DOU Leina, YAO Xiaolin, et al. Nanozyme amplification mediated on-demand multiplex lateral flow immunoassay with dual-readout and broadened detection range[J]. Biosensors & Bioelectronics,2020,169:112610.
[62] TIAN Meiling, XIE Wenyue, ZHANG Ting, et al. A sensitive lateral flow immunochromatographic strip with prussian blue nanoparticles mediated signal generation and cascade amplification[J]. Sensors and Actuators B: Chemical,2020,309:127728. doi: 10.1016/j.snb.2020.127728
[63] DUAN Demin, FAN Kelong, ZHANG Dexi, et al. Nanozyme-strip for rapid local diagnosis of Ebola[J]. Biosensors & Bioelectronics,2015,74:134−141.
[64] WEN Congying, HU Jun, ZHAGN Zhiling, et al. One-step sensitive detection of Salmonella typhimurium by coupling magnetic capture and fluorescence identification with functional nanospheres[J]. Analytical Chemistry,2013,85(2):1223−1230. doi: 10.1021/ac303204q
[65] HUAGN Yan, WEN Yongqiang, BARYEH K, et al. Magnetized carbon nanotubes for visual detection of proteins directly in whole blood[J]. Anal Chim Acta,2017,993:79−86. doi: 10.1016/j.aca.2017.09.025
[66] WU Min, ZHANG Zhiling, CHEN Gang, et al. Rapid and quantitative detection of avian influenza A (H7N9) virions in complex matrices based on combined magnetic capture and quantum dot labeling[J]. Small,2015,11(39):5280−5288. doi: 10.1002/smll.201403746
[67] SHARMAA A, TOK A I Y, LEE C, et al. Magnetic field assisted preconcentration of biomolecules for lateral flow assaying[J]. Sensors and Actuators B: Chemical,2019,285:431−437. doi: 10.1016/j.snb.2019.01.073
[68] TSAI T T, HUANG T H, CHEN C A, et al. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay[J]. Scientific Reports,2018,8(1):17319. doi: 10.1038/s41598-018-35694-9
[69] ZHANG Sufeng, LIU Lina, TANG Ruihua, et al. Sensitivity enhancement of lateral flow assay by embedding cotton threads in paper[J]. Cellulose,2019,26(13-14):8087−8099. doi: 10.1007/s10570-019-02677-6
[70] TANG Ye, GAO Hui, KURTH F, et al. Nanocellulose aerogel inserts for quantitative lateral flow immunoassays[J]. Biosensors and Bioelectronics,2012,192:113491.
[71] CHOI J R, LIU Zhi, HU Jie, et al. Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing[J]. Analytical Chemistry,2016,88(12):6254−6264. doi: 10.1021/acs.analchem.6b00195
[72] DANIELQ G, CHRISTINA S, ISRAEL G, et al. Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers[J]. Biosensors & Bioelectronics,2019,141:111407.
[73] AMADEO S T, NGO D B, PAROLO C, et al. Lateral flow assay modified with time-delay wax barriers as a sensitivity and signal enhancement strategy[J]. Biosensors and Bioelectronics,2020,168:112559. doi: 10.1016/j.bios.2020.112559
[74] KATIS I N, HE P J W, EASON R W, et al. Improved sensitivity and limit-of-detection of lateral flow devices using spatial constrictions of the flow-path[J]. Biosensors and Bioelectronics,2018,113:95−100. doi: 10.1016/j.bios.2018.05.001
[75] WU Zhengzong, HE Deyun, XU Enbo, et al. Rapid detection of beta-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification[J]. Food Chemistry,2018,269:375−379. doi: 10.1016/j.foodchem.2018.07.011
[76] CHOI J R, YONG K W, TANG Ruihua, et al. Lateral flow assay based on paper-hydrogel hybrid material for sensitive point-of-care detection of dengue virus[J]. Advanced Healthcare Materials,2017,6(1):1600920. doi: 10.1002/adhm.201600920
[77] RIVAS L, MEDINA-SANCHEZ M, DE LA ESCOSURA-MUÑIZ A, et al. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics[J]. Lab on a Chip,2014,14(22):4406−4414. doi: 10.1039/C4LC00972J
[78] TANG Ruihua, LIU Lina, ZHANG Sufeng, et al. Modification of a nitrocellulose membrane with cellulose nanofibers for enhanced sensitivity of lateral flow assays: Application to the determination of Staphylococcus aureus[J]. Microchimica Acta,2019,186(12):831. doi: 10.1007/s00604-019-3970-z
[79] WANG Yiru, QIN Zhengpeng, BOULWARE D R, et al. Thermal contrast amplification reader yielding 8-fold analytical improvement for disease detection with lateral flow assays[J]. Analytical Chemistry,2016,88(23):11774−11782. doi: 10.1021/acs.analchem.6b03406
[80] HU Xiaoyan, WAN Jiangshan, PENG Xiaole, et al. Calorimetric lateral flow immunoassay detection platform based on the photothermal effect of gold nanocages with high sensitivity, specificity, and accuracy[J]. International Journal of Nanomedicine,2019,14:7695−7705. doi: 10.2147/IJN.S218834
[81] QU Zhuo, WANG Kan, ALFRANCA G, et al. A plasmonic thermal sensing based portable device for lateral flow assay detection and quantification[J]. Nanoscale Research Letters,2020,15(1):10. doi: 10.1186/s11671-019-3240-3
[82] SU Lihong, CHEN Yaqian, WANG Lulu, et al. Dual-signal based immunoassay for colorimetric and photothermal detection of furazolidone[J]. Sensors and Actuators B: Chemical,2021,331:129431. doi: 10.1016/j.snb.2020.129431
[83] WANG Rui, KIM K, CHOI N, et al. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips[J]. Sensors and Actuators B: Chemical,2018,270:72−79.
[84] LI Yu, TANG Shusheng, ZHANG Wanjun, et al. A surface-enhanced Raman scattering-based lateral flow immunosensor for colistin in raw milk[J]. Sensors and Actuators B: Chemical,2019,282:703−711. doi: 10.1016/j.snb.2018.11.050
[85] SHENG E, LU Yuxiao, XIAO Yue, et al. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip[J]. Biosensors and Bioelectronics,2021,181:113149. doi: 10.1016/j.bios.2021.113149
[86] ZHANG Dan, DU Shuyuan, SU Shupeng, et al. Rapid detection method and portable device based on the photothermal effect of gold nanoparticles[J]. Biosensors and Bioelectronics,2019,123:19−24. doi: 10.1016/j.bios.2018.09.039
[87] WANG Chongwen, WANG Chaoguang, WANG Xiaolong, et al. Magnetic SERS strip for sensitive and simultaneous detection of respiratory viruses[J]. ACS Applied Materials & Interfaces,2019,11(21):19495−19505.
[88] YADAV S, SADIQUE M A, RANJAN P, et al. SERS based lateral flow immunoassay for point-of-Care detection of SARS-CoV-2 in clinical samples[J]. ACS Applied Bio Materials,2021,4(4):2974−2995. doi: 10.1021/acsabm.1c00102
[89] XIAO Rui, LU Luchun, RONG Zhen, et al. Portable and multiplexed lateral flow immunoassay reader based on SERS for highly sensitive point-of-care testing[J]. Biosensors and Bioelectronics,2020,168:112524. doi: 10.1016/j.bios.2020.112524
[90] LIN L K, STANCIU L A. Bisphenol A detection using gold nanostars in a SERS improved lateral flow immunochromatographic assay[J]. Sensors and Actuators B: Chemical,2018,276:222−229. doi: 10.1016/j.snb.2018.08.068
[91] SU Lihong, HU Huilan, TIAN Yanli, et al. Highly sensitive colorimetric/surface-enhanced raman spectroscopy immunoassay relying on a metallic core-shell Au/Au nanostar with clenbuterol as a target analyte[J]. Analytical Chemistry,2021,93(23):8362−8369. doi: 10.1021/acs.analchem.1c01487





 下载:
下载:





 下载:
下载:
