GC-MS Analysis of Constituents of Volatile Oil in Different Parts of Vitex negundo var. heterophylla
-
摘要: 目的:比较荆条茎、叶和花中挥发油成分的差异。方法:采用水蒸气蒸馏法分别提取荆条不同部位中的挥发油,运用气相色谱-质谱法(GC-MS)法对其组成成分进行分析鉴定,采用主成分分析和聚类分析其成分的差异。结果:从荆条不同部位共鉴定出83种挥发性成分,包括烯烃类、醇类、酮类、酯类等。茎、叶和花中分别鉴定出14、58、55种,占各部位挥发油总量的59.91%、98.32%和95.83%。3个部位共有成分仅有8种,但茎、叶和花中特有成分分别为4,22和21种。通过主成分分析得到荆条不同部位挥发油的差异成分,其中,茎中标志性挥发性成分为2,4-二叔丁基苯酚、14-甲基十五烷酸甲酯、正二十一烷等,叶中标志性挥发性成分为β-松油烯、榄香醇、丁香烯氧化物等,而花中标志性挥发性成分是由桧烯、2,3-二氢-3-[2-氨基乙基]-5-甲氧基-1,3-二甲基吲哚-2-酮、二苯环庚烯酰胺等组成。由聚类分析结果可知,荆条茎与花聚为一类,再与叶聚为一类。结论:荆条茎、叶和花中挥发油成分的种类和含量有一定的差异性,这为荆条不同部位进一步药用或工业开发提供科学依据。
-
关键词:
- 荆条 /
- 挥发油 /
- 气相色谱-质谱法(GC-MS) /
- 主成分分析 /
- 聚类分析
Abstract: Objective: The study aimed to compare the variance of constituents of volatile oil in the stems, leaves and flowers of Vitex negundo var. heterophylla (VNH). Methods: The volatile oil in the stems, leaves and flowers of VNH were extracted by hydrodistillation, respectively, and their constituents were analyzed and identified by GC-MS, and the differences in their chemical compositions were analyzed by principal component analysis (PCA) and cluster analysis. Results: Total of 83 volatile components were identified from different parts of the VNH, including olefins, alcohols, ketones, esters, etc. 14, 58 and 55 volatile components were detected from the stems, leaves, and flowers, accounting for 59.91%, 98.32% and 95.83% of the total volatile oils in each part, respectively. Only 8 volatile components were shared by three parts, whereas 4, 22 and 21 volatile components were unique to the stems, leaves and flowers, respectively. The differential components of the volatile oils from different parts of VNH were obtained by PCA, in which the signature volatile components in the stem were 2,4-bis (1,1-dimethylethyl)-phenol, 14-methyl-pentadecanoic acid, methyl ester, heneicosane, etc, and the trademark volatile components in the leaves were β-terpinene, elemol, caryophyllene oxide, etc, whereas the hallmark volatile components in the flowers were formed by sabenene, 2,3-dihydro-3-[2-aminoethyl]-5-methoxy-1,3- dimethyl-indole-2-one, citenamide, etc. As revealed by the results of the cluster analysis, the stems of VNH were clustered with the flowers and then with the leaves. Conclusion: The composition of the volatile oil in the stems, leaves and flowers of of VNH had certain variability, which would provide a scientific basis for the further medicinal or industrial exploitation of different parts of VNH. -
荆条(Vitex negundo var. heterophylla)是黄荆的主要变种,系马鞭草科牡荆属多年生小灌木,分布于我国东北、华中、西北等地区。在我国民间多当草药使用,其茎叶可治久痢,而花、枝叶可作为提取植物芳香油的原料[1]。此外,荆条具有较高的经济价值,花可作为优良的蜜源,叶片可泡茶饮用,也可开发成具抗疲劳、提神醒脑等功效的功能性食品[2-3]。现代研究表明,荆条主要含有木脂素、类黄酮、环烯醚萜类、倍半萜类和三萜类等成分,具有显著的抗炎、抗氧化的药理活性[4-8]。
目前,关于荆条的挥发油的研究多集中于GC-MS分析荆条叶和花中挥发油组成成分。刘晶鑫等[8]和王发松等[9]从荆条叶的挥发油中发现β-丁香烯含量较高。张书锋等[10]发现野生荆条花挥发油的主要化学成分为β-丁香烯、β-水芹烯和桉油精等。这些研究对于了解荆条叶和花中挥发油成分有一定的帮助,但忽略了荆条茎挥发油成分的研究,未能全面分析荆条茎、叶和花之间挥发油成分组成与含量差异。因此,本实验采用GC-MS技术对荆条不同部位中挥发性组成成分进行比较,旨在为明确荆条不同部位的物质基础和荆条的合理开发利用提供科学支撑。
1. 材料与方法
1.1 材料与仪器
荆条 采自河南省辉县市,于2021年7月花开放后采集;无水乙醇、石油醚、无水硫酸钠 分析纯,国药集团化学试剂有限公司。
FA2004电子天平 上海良平仪器仪表有限公司;1200型手掌离心机 江苏新康医疗器械有限公司;安捷伦7890B 7000C气质联用仪、Agilent 19091S-433色谱柱(30 m×250 μm×0.25 μm) 美国Agilent公司。
1.2 实验方法
1.2.1 材料预处理
将新鲜的荆条按照茎、叶、花三个部位进行裁剪、洗净、阴干、粉碎,过65目筛,备用。
1.2.2 挥发油的提取
称取荆条茎、叶、花粗粉各50 g,置1000 mL圆底烧瓶中,按照料液比1:10 mL/g加入蒸馏水,室温浸泡2 h,按照2020 版《中国药典》四部附录中“挥发油测定法”中的“甲法”提取10 h,静置冷却分层,得淡黄色油状物。用石油醚溶解油状物置离心管中,再加无水硫酸钠,于12000 r·min−1离心30 min,吸取上清液,备用。
1.2.3 GC-MS分析
色谱条件:Agilent 19091S-433色谱柱(30 m×250 μm×0.25 μm),载气为氦气,流量2.3145 mL/min,进样量为0.2 μL,不分流,进样口温度250 ℃;程序升温条件:初始温度60 ℃,保持1 min,然后以12 ℃·min−1升至200 ℃后,再以5 ℃·min−1升至280 ℃·min−1,保持4 min。
质谱条件:离子源EI,电子能量70 eV,离子源温度250 ℃,质量范围m/z 40~500。
1.3 数据处理
采用NIST 10标准质谱图库和文献资料分析荆条茎、叶和花的挥发油成分总离子流图确定其组成,根据峰面积归一化法计算各挥发性成的相对百分含量[11]。采用SIMCA 14.1软件和微生信在线平台(http://www.bioinformatics.com.cn)对荆条不同部位的挥发油成分进行主成分分析和聚类分析。
2. 结果与分析
2.1 荆条不同部位挥发油成分分析
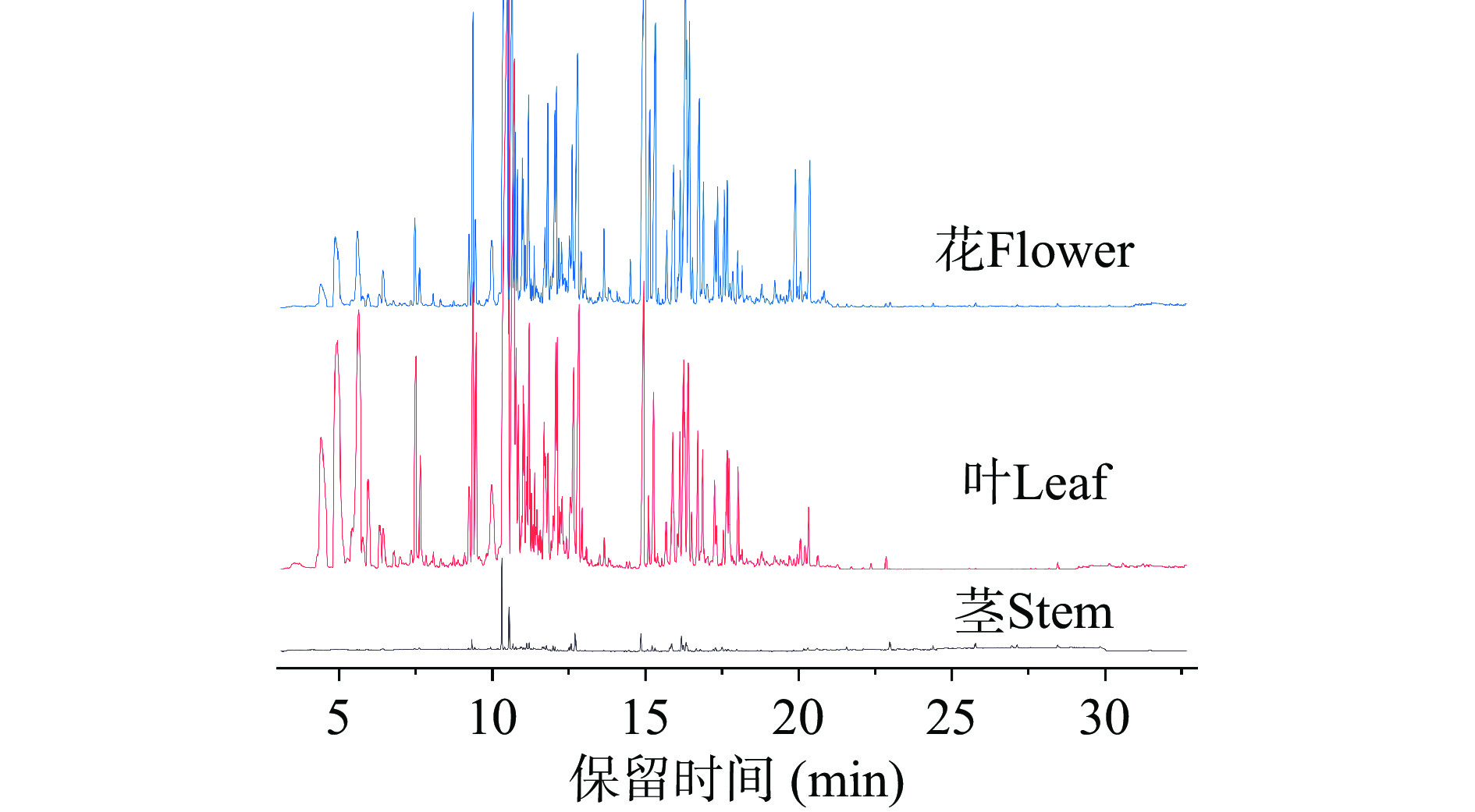
荆条茎、叶和花中挥发油的总离子流图如图1,按照面积归一法计算各挥发油成分相对百分含量,结果见表1。
由表1可知,从荆条不同部位中的挥发油成分共鉴定出83种,包括烯烃类34种,酮类12种、醇类16种、氧化物类和烷烃类各3种、醚类、酯类和醛类各2种、其他10种。荆条茎、叶和花中分别鉴定出14、58和55种,相对含量分别占其总挥发油的59.91%、98.32%和95.83%。
表 1 荆条不同部位挥发油成分Table 1. Volatile components from different parts of VNH种类 编号 化合物名称 分子量 相对百分含量(%) 茎 叶 花 烯烃类 1 α-蒎烯 136.125 − 4.52 0.91 2 桧烯 136.125 − − 2.57 3 β-松油烯 136.125 − 8.20 − 4 γ-松油烯 136.125 − 1.25 0.20 5 异松油烯 136.125 − 0.53 0.18 6 2,6-二甲基-2,4,6-辛三烯 136.125 − 0.20 − 7 γ-Pyronene 136.125 1.96 0.70 0.65 8 δ-Pyronene 136.125 − 2.79 2.9 9 4-蒈烯 136.125 − 2.10 0.65 10 β-榄香烯 204.188 − 1.80 1.43 11 β-丁香烯 204.188 19.12 17.98 16.61 12 β-金合欢烯 204.188 8.77 8.44 5.73 13 律草烯 204.188 − 1.87 1.64 14 异香树烯 204.188 − 1.16 0.78 15 α-榄香烯 204.188 − 1.64 0.87 16 大根香叶烯D 204.188 − − 0.57 17 α-芹子烯 204.188 − − 0.31 18 大根香叶烯B 204.188 − 1.75 1.85 19 δ-杜松烯 204.188 − 0.46 0.33 20 雅槛兰树油烯 204.188 − − 1.56 21 β-芹子烯 204.188 − 0.37 − 22 3,7(11)-桉叶二烯 204.188 1.11 0.75 0.65 23 6,6-二甲基庚烷-2,4-二烯 124.125 − 0.18 0.45 24 Guaia-3,9-diene 204.188 − 0.43 − 25 δ-愈创木烷 204.188 − − 0.60 26 新瑟模环烯A 272.25 − 0.19 − 27 2,2,4,8-四甲基三环[5.3.1.0(4,11)]十一碳八烯 204.188 4.19 2.07 7.10 28 4(14),11 -桉叶二烯 204.188 − 0.75 − 29 γ-雪松烯 204.188 − 0.25 0.96 30 4-甲基-1-甲基-2-(2-甲基-1-1烯丙基)-1-乙烯基-环庚烷 204.188 − 0.26 − 31 6,10-二甲基-3-(1-甲基亚乙基)-1-环癸烯 206.203 − − 0.33 32 1,1-二甲基-2,4-双(1-甲基乙烯基)-环己烷 192.188 − 0.16 − 33 1,3-dimethyl-2-(1-methylethylidene)-Cyclopentane 138.141 − − 0.23 34 角鲨烯 410.391 − 0.05 − 酮类 35 6,10,14-三甲基-2-十五烷酮 268.277 − − 0.34 36 β-甲基紫罗兰酮 206.167 4.67 3.09 10.27 37 7-羟基-2,5,8-喹啉三酮 191.022 1.65 1.48 − 38 2,3-二氢-3-[2-氨基乙基]-5-甲氧基-1,3-二甲基吲哚-2-酮 234.137 − − 3.97 39 4-甲基-5-(1-丁烯-2-基)-5-(2-甲基-2-丙烯基)-2-呋喃酮 206.131 − 0.38 − 40 1,3,3-trimethyl-2-(1-methylbut-1-en-3-on-1-yl)-1-Cyclohexene 206.167 − 1.48 2.26 41 1,3-二甲基蝶啶-2,4-二酮 192.065 − 1.02 − 42 3,3-二甲基-2-[(1E)-3-甲基-1,3-丁二烯基]环戊酮 178.136 − − 1.50 43 4,6-甾二烯-3-酮 270.162 − 1.05 1.21 44 11β-羟基雄烯二酮 302.188 − − 1.58 45 7-羟基-1-茚酮 148.052 − 0.25 − 46 (4S,5R)-5-Methylspiro[3.5]nonan-1-one 152.12 − 0.46 1.87 醇类 47 桉树脑 154.136 − 6.91 1.71 48 芳樟醇 154.136 − 0.48 0.59 49 β-松油醇 154.136 − 0.17 − 50 α-松油醇 154.136 − 0.93 0.38 51 松油烯-4-醇 154.136 − 2.53 0.99 52 顺式辣薄荷醇 154.136 − 0.10 − 53 香叶醇 154.136 − − 0.07 54 榄香醇 222.198 − 2.01 − 55 橙花叔醇 222.198 − − 0.97 56 匙叶桉油烯醇 220.183 − 1.7 1.55 57 绿花白千层醇 222.198 − − 0.32 58 茅苍术醇 222.198 2.03 1.99 1.45 59 β-桉叶醇 222.198 5.2 3.15 3.94 60 没药醇 222.198 − − 1.75 61 叶绿醇 296.308 − 0.92 − 62 2-甲基-2-(4-甲基-3-戊烯基)-环丙烷甲醇 168.151 − − 0.28 酯类 63 14-甲基十五烷酸甲酯 270.256 0.67 − − 64 邻苯二甲酸,3,5-二甲苯基4型苯乙烯酯 374.115 − 0.86 − 醛类 65 长叶醛 220.183 − 0.37 − 66 3-环己基-2-甲基-丙醛 152.12 − 0.23 − 醚类 67 细辛醚 208.11 − 0.64 1.47 68 泪柏醚 290.261 − 0.39 0.36 氧化物类 69 丁香烯氧化物 220.183 − 1.12 − 70 顺-Z-α-红没药烯环氧化物 220.183 − − 0.44 71 氧化喇叭茶烯-(II) 220.183 − 0.10 − 烷烃类 72 Dehydroabietan 270.235 − 0.74 0.92 73 正二十一烷 296.344 3.45 − − 其他 74 1,2,3-三甲基-2-环戊烯-1-羧酸 154.099 − 0.10 0.08 75 2,4-二叔丁基苯酚 206.167 1.81 − − 76 N-(2-氧代-1,3-二氢苯并咪唑-5-基)乙酰胺 191.069 2.75 − − 77 4-amino-6,7-dimethyl-1H-Pyrrolo[3,4-c]pyridine-1,3,(2H)-dione 204.188 2.53 1.94 − 78 二苯环庚烯酰胺 235.1 − − 3.30 79 5-甲基-2-烯丙基苯酚 148.089 − 0.77 1.01 80 N-(Chroman-7-yl)acetamide 191.095 − − 0.27 81 1-t-Butyl-4-(adamantyl-1)benzene 268.219 − − 0.64 82 6-Methyl-6,7-dihydro-9H-5-oxa-9-azabenzocyclohepten-8-one 177.079 − − 0.28 83 5-溴-1-甲基吲哚-2-羧酸 252.974 − 0.12 − 注:相对含量中“−”表示未检出该成分。 2.2 挥发油成分类别
为了确定荆条中不同部位主要贡献成分的类型,根据化合物的官能团将挥发油成分进行分类,并计算出各类化合物的相对百分含量,结果见表2。荆条茎、叶和花中所含主要化合物有烯烃类(35.15%、60.85%和50.06%)和醇类(7.23%、20.89%和14.00%),不同部位中烯烃类均以β-丁香烯(19.12%)占绝对主导;茎和花中醇类以β-桉叶醇(5.2%和3.94%)为主要贡献成分,而叶中则以桉树脑(6.91%)为主要成分。另外,荆条叶和花中烯烃类和醇类化合物的含量和数量均明显高于其茎。由表2可看出荆条不同部位主要挥发油成分组成相似,但在挥发油成分组成和含量上仍存在一定的差异。
表 2 荆条不同部位各挥发油成分的含量及数量Table 2. Various volatile components and quantities in different part of VN部位 烯烃类 酮类 醇类 酯类 醛类 醚类 氧化合物 烷烃类 其他 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 茎 35.15 5 6.32 2 7.23 2 0.67 1 0 0 0 0 0 0 3.45 1 7.09 3 叶 60.85 27 9.20 8 20.89 11 0.86 1 0.60 2 1.03 2 1.22 2 0.74 1 2.93 4 花 50.06 25 23.00 8 14.00 12 0 0 0 0 1.83 2 0.44 1 0.92 1 5.58 6 2.3 共有成分和特有成分
荆条茎、叶和花中所含共有挥发油成分有8种,包括γ-pyronene(7)、β-丁香烯(11)、β-金合欢烯(12)和3,7(11)-桉叶二烯(22)等。由图2可知,荆条不同部位的共同成分的相对含量存在较大差异,其中β-丁香烯在荆条不同部位中含量都较高,β-丁香烯是一种萜烯类成分,在抗肿瘤、镇痛、神经保护、抗菌、抗炎和抗氧化等方面产生显著的生物活性[12-17]。β-金合欢烯(12)的含量茎(8.77%)>叶(8.44%)>花(5.73%),β-金合欢烯调节H2O2诱导的氧化应激神经毒性和遗传毒性[18];另外,β-金合欢烯是多种蚜虫虫害的警报信息素[19],因此,可通过吸引蚜虫捕食者来作为间接防御[20-21]。β-甲基紫罗兰酮(36)的含量花 (10.27%) 远高于其茎(4.67%)和叶(3.09%),可作为食用香料和日用香精。茎中茅苍术醇(58)和β-桉叶醇(59)的含量高于其花和叶,β-桉叶醇具有潜在的抗过敏、抗肿瘤和抗血管生成活性[22-25]。茅苍术醇能够抑制肿瘤细胞的增殖和凋亡而发挥其抗肿瘤活性[26-27]。
从荆条不同部位中挥发油成分的组差异分析,荆条花中特有成分有21种,占其挥发油成分量的21.88%,包括烯烃类7种(6.17%),酮类4种(7.39%),醇类5种(3.39%),氧化物类1种(0.44%),其他4种(4.49%),主要特有成分有2,3-二氢-3-[2-氨基乙基]-5-甲氧基-1,3-二甲基吲哚-2-酮(3.97%)、二苯环庚烯酰胺(3.3%)、桧烯(2.57%)等。荆条叶中特有成分22种,占其挥发油成分的18.26%,包括烯烃类9种(10.61%)、酮类3种(1.65%)、醇类4种(3.2%)、醛类2种(0.6%)、氧化物类2种(1.22%)、酯类1种(0.86%)、其他1种(0.12%),主要特有成分有β-松油烯(8.2%)、榄香醇(2.01%)、丁香烯氧化物(1.12%)等。荆条茎中特有挥发油成分4种,占其挥发油成分的8.68%,包括正二十一烷(3.45%)、N-(2-氧代-1,3-二氢苯并咪唑-5-基)乙酰胺(2.75%)、2,4-二叔丁基苯酚(1.81%)、14-甲基十五烷酸甲酯(0.67%)。
2.4 主成分分析
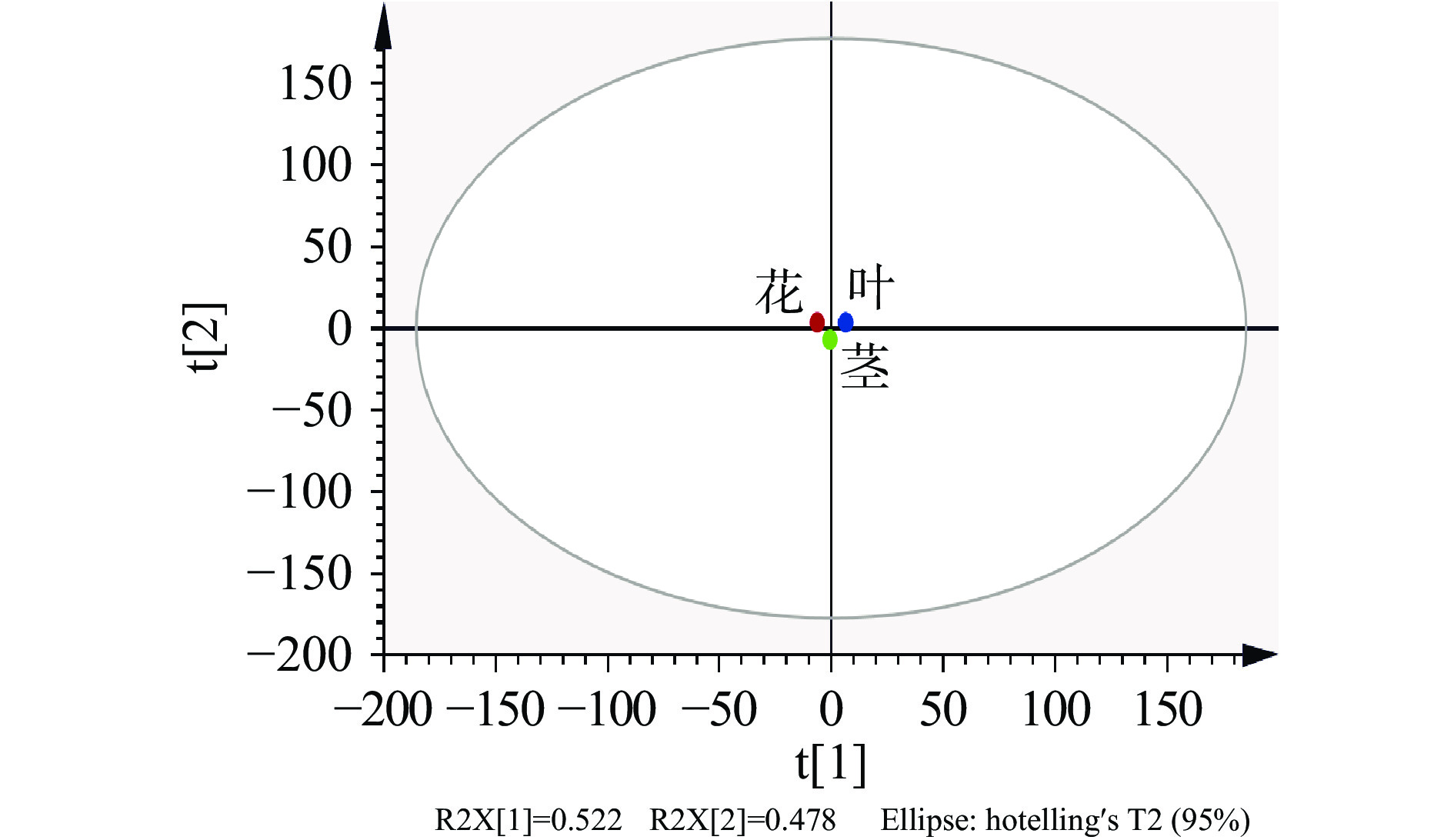
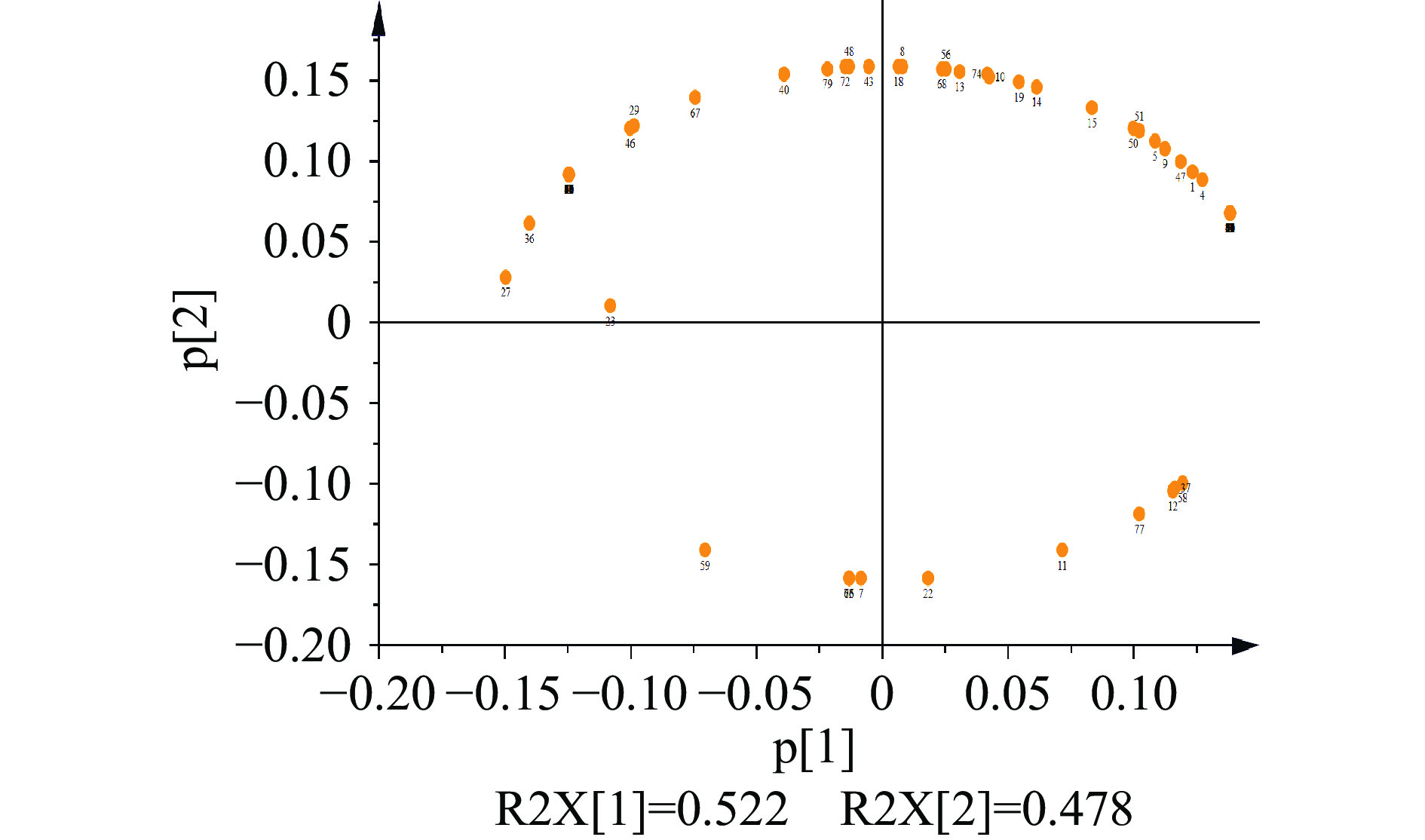
为了直观、判断荆条不同部位之间挥发油成分是否存在差异,采用Smica-P软件对荆条不同部位挥发性进行主成分分析,具体结果见得分图3和载荷图4。得分图是通过分析不同样品之间的距离判断各样品挥发油组成成分的差异程度,载荷图根据不同化合物与原点之间的距离和方向确定各种挥发油成分与各个主成分之间的相关性[28]。
由图3可知,第1和2主成分的方差贡献率分别为52.2%和47.8%,累计方差贡献率达到100%,能全面反映样品的整体信息。因此,以上两个主成分可作为数据分析的有效成分。在得分图中显示荆条不同部位处于相对独立的空间,表明荆条不同部位挥发油成分有显著差别,同时说明PCA分析能够对荆条不同部位进行有效的区分。此外,图中花和茎中距离较近,二者与叶距离较远,说明花和茎中挥发油的组成和含量有一定的相似性,而与叶有较大差异。
由图4可知,各个挥发油成分在载荷图中中各象限的分布较为分散,说明差异明显的相关物质在主成分中的贡献率比较大。载荷图中各个挥发油成分与原点的距离越远,该成分对应主成分的相关性越高,说明这些化合物可能就是区分不同部位的关键化合物[29]。β-松油烯(3)、榄香醇(54)、丁香烯氧化物(69)等挥发油成分,分布于第一象限内,与PC1呈正相关,是荆条叶的主要差异成分。桧烯(2)、2,3-二氢-3-[2-氨基乙基]-5-甲氧基-1,3-二甲基吲哚-2-酮(38)、二苯环庚烯酰胺(78)等物质位于第二象限,与PC1呈负相关,是花的主要差异性成分。茎的差异性成分位于第三、四象限内,与PC2呈负相关,其中贡献较大的包括2,4-二叔丁基苯酚(75)、14-甲基十五烷酸甲酯(63)、正二十一烷(73)等。以上差异性成分可作为区分荆条不同部位的化学标志物。
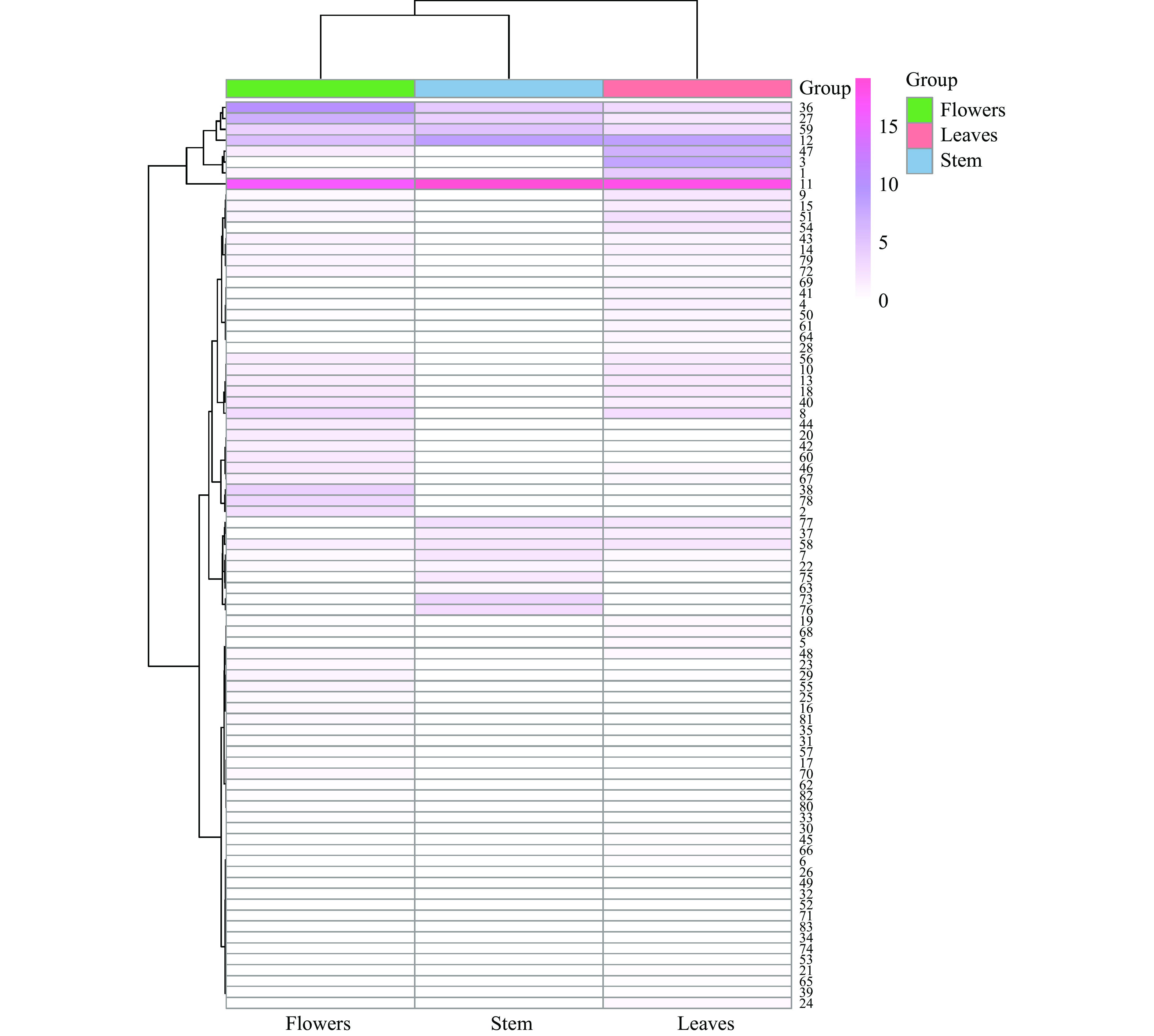
2.5 聚类分析
采用Ward聚类法,以Euclidean距离为测度,相对百分含量为基准,对荆条不同部位的挥发油成分进行聚类分析[30],结果见图5。上方树状表示荆条不同部位的聚类,中部填充颜色的深浅表示各部位中各类挥发油成分含量的高低,颜色越深表示相对含量越高。由上方的聚类分析可知,荆条的花和茎聚为一类,再与叶聚为一类,说明荆条花和茎挥发油成分种类和含量间有一定的相似度,但与叶之间的差别较大,这与主成分分析的结果保持一致。
3. 结论
本实验采用GC-MS联用分析技术从荆条茎、叶和花中的挥发油中共鉴定出83种挥发性成分,其中,从茎中鉴定出14种,叶中鉴定出58种,花中分鉴定出55种。通过PCA和聚类分析发现荆条不同部位的挥发油组成成分和含量存在一定的差异。从种类的角度来看,荆条不同部位挥发性成分中既有共有成分,也存在特有成分。共有挥发性成分仅有8种,且相对含量存在较大差异。其中,β-丁香烯在荆条茎、叶和花中相对含量较高,分别为19.12%,17.98%和16.61%。另外,从分类的角度来看,荆条茎、叶和花中烯烃类化合物相对含量较高,而烯烃类主要成分中β-丁香烯和β-金合欢烯的含量较高。β-丁香烯在抗肿瘤、镇痛、神经保护、抗菌、抗炎等方面显示较好的药理活性,而β-金合欢烯可以作为某些香料产品的基本原料。因此,无论是在临床上,还是在农业或者工业生产中,荆条茎挥发性成分的加工和开发都是不可忽视的。综上所述,荆条不同部位挥发油具有较高药用和工业价值,这为荆条不同部位挥发油的合理应用提供理论依据。
-
表 1 荆条不同部位挥发油成分
Table 1 Volatile components from different parts of VNH
种类 编号 化合物名称 分子量 相对百分含量(%) 茎 叶 花 烯烃类 1 α-蒎烯 136.125 − 4.52 0.91 2 桧烯 136.125 − − 2.57 3 β-松油烯 136.125 − 8.20 − 4 γ-松油烯 136.125 − 1.25 0.20 5 异松油烯 136.125 − 0.53 0.18 6 2,6-二甲基-2,4,6-辛三烯 136.125 − 0.20 − 7 γ-Pyronene 136.125 1.96 0.70 0.65 8 δ-Pyronene 136.125 − 2.79 2.9 9 4-蒈烯 136.125 − 2.10 0.65 10 β-榄香烯 204.188 − 1.80 1.43 11 β-丁香烯 204.188 19.12 17.98 16.61 12 β-金合欢烯 204.188 8.77 8.44 5.73 13 律草烯 204.188 − 1.87 1.64 14 异香树烯 204.188 − 1.16 0.78 15 α-榄香烯 204.188 − 1.64 0.87 16 大根香叶烯D 204.188 − − 0.57 17 α-芹子烯 204.188 − − 0.31 18 大根香叶烯B 204.188 − 1.75 1.85 19 δ-杜松烯 204.188 − 0.46 0.33 20 雅槛兰树油烯 204.188 − − 1.56 21 β-芹子烯 204.188 − 0.37 − 22 3,7(11)-桉叶二烯 204.188 1.11 0.75 0.65 23 6,6-二甲基庚烷-2,4-二烯 124.125 − 0.18 0.45 24 Guaia-3,9-diene 204.188 − 0.43 − 25 δ-愈创木烷 204.188 − − 0.60 26 新瑟模环烯A 272.25 − 0.19 − 27 2,2,4,8-四甲基三环[5.3.1.0(4,11)]十一碳八烯 204.188 4.19 2.07 7.10 28 4(14),11 -桉叶二烯 204.188 − 0.75 − 29 γ-雪松烯 204.188 − 0.25 0.96 30 4-甲基-1-甲基-2-(2-甲基-1-1烯丙基)-1-乙烯基-环庚烷 204.188 − 0.26 − 31 6,10-二甲基-3-(1-甲基亚乙基)-1-环癸烯 206.203 − − 0.33 32 1,1-二甲基-2,4-双(1-甲基乙烯基)-环己烷 192.188 − 0.16 − 33 1,3-dimethyl-2-(1-methylethylidene)-Cyclopentane 138.141 − − 0.23 34 角鲨烯 410.391 − 0.05 − 酮类 35 6,10,14-三甲基-2-十五烷酮 268.277 − − 0.34 36 β-甲基紫罗兰酮 206.167 4.67 3.09 10.27 37 7-羟基-2,5,8-喹啉三酮 191.022 1.65 1.48 − 38 2,3-二氢-3-[2-氨基乙基]-5-甲氧基-1,3-二甲基吲哚-2-酮 234.137 − − 3.97 39 4-甲基-5-(1-丁烯-2-基)-5-(2-甲基-2-丙烯基)-2-呋喃酮 206.131 − 0.38 − 40 1,3,3-trimethyl-2-(1-methylbut-1-en-3-on-1-yl)-1-Cyclohexene 206.167 − 1.48 2.26 41 1,3-二甲基蝶啶-2,4-二酮 192.065 − 1.02 − 42 3,3-二甲基-2-[(1E)-3-甲基-1,3-丁二烯基]环戊酮 178.136 − − 1.50 43 4,6-甾二烯-3-酮 270.162 − 1.05 1.21 44 11β-羟基雄烯二酮 302.188 − − 1.58 45 7-羟基-1-茚酮 148.052 − 0.25 − 46 (4S,5R)-5-Methylspiro[3.5]nonan-1-one 152.12 − 0.46 1.87 醇类 47 桉树脑 154.136 − 6.91 1.71 48 芳樟醇 154.136 − 0.48 0.59 49 β-松油醇 154.136 − 0.17 − 50 α-松油醇 154.136 − 0.93 0.38 51 松油烯-4-醇 154.136 − 2.53 0.99 52 顺式辣薄荷醇 154.136 − 0.10 − 53 香叶醇 154.136 − − 0.07 54 榄香醇 222.198 − 2.01 − 55 橙花叔醇 222.198 − − 0.97 56 匙叶桉油烯醇 220.183 − 1.7 1.55 57 绿花白千层醇 222.198 − − 0.32 58 茅苍术醇 222.198 2.03 1.99 1.45 59 β-桉叶醇 222.198 5.2 3.15 3.94 60 没药醇 222.198 − − 1.75 61 叶绿醇 296.308 − 0.92 − 62 2-甲基-2-(4-甲基-3-戊烯基)-环丙烷甲醇 168.151 − − 0.28 酯类 63 14-甲基十五烷酸甲酯 270.256 0.67 − − 64 邻苯二甲酸,3,5-二甲苯基4型苯乙烯酯 374.115 − 0.86 − 醛类 65 长叶醛 220.183 − 0.37 − 66 3-环己基-2-甲基-丙醛 152.12 − 0.23 − 醚类 67 细辛醚 208.11 − 0.64 1.47 68 泪柏醚 290.261 − 0.39 0.36 氧化物类 69 丁香烯氧化物 220.183 − 1.12 − 70 顺-Z-α-红没药烯环氧化物 220.183 − − 0.44 71 氧化喇叭茶烯-(II) 220.183 − 0.10 − 烷烃类 72 Dehydroabietan 270.235 − 0.74 0.92 73 正二十一烷 296.344 3.45 − − 其他 74 1,2,3-三甲基-2-环戊烯-1-羧酸 154.099 − 0.10 0.08 75 2,4-二叔丁基苯酚 206.167 1.81 − − 76 N-(2-氧代-1,3-二氢苯并咪唑-5-基)乙酰胺 191.069 2.75 − − 77 4-amino-6,7-dimethyl-1H-Pyrrolo[3,4-c]pyridine-1,3,(2H)-dione 204.188 2.53 1.94 − 78 二苯环庚烯酰胺 235.1 − − 3.30 79 5-甲基-2-烯丙基苯酚 148.089 − 0.77 1.01 80 N-(Chroman-7-yl)acetamide 191.095 − − 0.27 81 1-t-Butyl-4-(adamantyl-1)benzene 268.219 − − 0.64 82 6-Methyl-6,7-dihydro-9H-5-oxa-9-azabenzocyclohepten-8-one 177.079 − − 0.28 83 5-溴-1-甲基吲哚-2-羧酸 252.974 − 0.12 − 注:相对含量中“−”表示未检出该成分。 表 2 荆条不同部位各挥发油成分的含量及数量
Table 2 Various volatile components and quantities in different part of VN
部位 烯烃类 酮类 醇类 酯类 醛类 醚类 氧化合物 烷烃类 其他 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 含量(%) 数量 茎 35.15 5 6.32 2 7.23 2 0.67 1 0 0 0 0 0 0 3.45 1 7.09 3 叶 60.85 27 9.20 8 20.89 11 0.86 1 0.60 2 1.03 2 1.22 2 0.74 1 2.93 4 花 50.06 25 23.00 8 14.00 12 0 0 0 0 1.83 2 0.44 1 0.92 1 5.58 6 -
[1] 中国科学院《中国植物志》编辑委员会. 中国植物志[M]. 北京: 科学出版社, 1982, 65: 131, 145. Editorial Board of Flora of China, Chinese Academy of Sciences. Flora of China[M]. Beijing: Science Press, 1982, 65: 131, 145.
[2] GILL B S, MEHRA R, KUMAR N S. Vitex negundo and its medicinal value[J]. Molecular Biology Reports, 2018, 45: 2925−2934.
[3] ZHENG C J, LI H Q, REN S C, et al. Phytochemical and pharmacological profile of Vitex negundo[J]. Phytotherapy Research, 2015, 29: 633−647.
[4] XU J M, HU B C, YUAN L, et al. Labdanes and megastigmanes from Vitex negundo var. heterophylla[J]. Fitoterapia,2019,137:104265−104273. doi: 10.1016/j.fitote.2019.104265
[5] HUANG Y, DING G Y, HU P. Vitexnegheteroin M, a new phenolic glycoside from Vitex negundo var. heterophylla[J]. Natural Product Research, 2021, 35(9): 1518−1524.
[6] QIU C, TONG L, YUAN T, et al. Constituents from Vitex negundo var. heterophylla and their inhibition of nitric oxide production[J]. Journal of Natural Medicines,2017,71(1):292−298. doi: 10.1007/s11418-016-1032-y
[7] HU P, LI D H, HU X, et al. Lignans and triterpenoids from Vitex negundo var. heterophylla and their biological evaluation[J]. Fitoterapia,2016,111:147−53. doi: 10.1016/j.fitote.2016.04.020
[8] 刘晶鑫, 谢建春, 孙宝国, 等. 荆条挥发油中β-丁香烯的提取分离[J]. 食品与发酵工业,2007,33(10):168−170. [LIU Jingxin, XIE Jianchun, SUN Baoguo, et al. Extraction and separation of p-caryophyllene from leaf oil of Vitex negundo L. var. heterophyl (Franch.) Rehd[J]. Food and Fermentation Industries,2007,33(10):168−170. LIU Jingxin, XIE Jianchun, SUN Baoguo, et al. Extraction and separation of p-caryophyllene from leaf oil of Vitex negundo L. var. heterophyl (Franch. ) Rehd[J]. Food and Fermentation Industries, 2007, 33(10): 168-170.
[9] 王发松, 任三香, 杨得坡, 等. 荆条叶挥发油的气相色谱-质谱分析[J]. 质谱学报,2004,25(1):61−64. [WANG Fasong, REN Sanxiang, YANG Depo, et al. Determination of the volatile oil extracted from leaves of Vitex negundo var. heterophylla Rehd. by gas chromatograohy-mass spectrometry[J]. Journal of Chinese Mas Spectrometry Society,2004,25(1):61−64. doi: 10.3969/j.issn.1004-2997.2004.01.013 WANG Fasong, REN Sanxiang, YANG Depo, et al. Determination of the volatile oil extracted from leaves of Vitex negundo var. heterophylla Rehd. by gas chromatograohy-mass spectrometry[J]. Journal of Chinese Mas Spectrometry Society, 2004, 25(1): 61-64. doi: 10.3969/j.issn.1004-2997.2004.01.013
[10] 张书锋, 董庆峰, 胡聪, 等. 石家庄野生荆条花挥发油的化学成分[J]. 中国实验方剂学杂志,2013,19(23):151−153. [ZHANG Shufeng, DONG Qingfeng, HU Cong, et al. Chemical composition of flower volatile oil of wild Vitex negundo var. heterophylla from Shijiazhuang[J]. Chinese Journal of Experimental Traditional Medical Formulae,2013,19(23):151−153. ZHANG Shufeng, DONG Qingfeng, HU Cong, et al. Chemical composition of flower volatile oil of wild Vitex negundo var. heterophylla from Shijiazhuang[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2013, 19(23): 151-153.
[11] 杨翠, 赵锦花. GP-MSE/GC-MS分析夏枯草不同部位挥发性成分[J]. 食品工业科技,2019,40(13):180−185,192. [YANG Cui, ZHAO Jinhua. Analysis of volatile components from different parts of Prunella vulgaris by GP-MSE/GC-MS[J]. Science and Technology of Food Industry,2019,40(13):180−185,192. YANG Cui, ZHAO Jinhua. Analysis of volatile components from different parts of Prunella vulgaris by GP-MSE/GC-MS[J]. Science and Technology of Food Industry, 2019, 40(13): 180-185, 192.
[12] FIDYT K, FIEDOROWICZ A, STRZADALA L, et al. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties[J]. Cancer Medicine,2016,5(10):3007−3017. doi: 10.1002/cam4.816
[13] ARUL S, RAJAGOPALAN H, RAVI J, et al. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis[J]. Anti-Cancer Agents in Medicinal Chemistry,2020,20(13):1530−1537. doi: 10.2174/1871520620666200227093216
[14] KOYAMA S, PURK A, KAUR M, et al. Beta-caryophyllene enhances wound healing through multiple routes[J]. PLoS One,2019,14(12):1−32.
[15] MACHADO K D C, ISLAM M T, ALI E S, et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene[J]. Phytotherapy Research,2018,32(12):2376−2388. doi: 10.1002/ptr.6199
[16] MOO C L, YANG S K, OSMAN M A, et al. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus[J]. Polish Journal of Microbiology,2020,69(1):1−6.
[17] LI H, WANG D, CHEN Y, et al. β-Caryophyllene inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells[J]. International Immunopharmacology,2020,84:106556−106564. doi: 10.1016/j.intimp.2020.106556
[18] TURKEZ H, SOZIO P, GEYIKOGLU F, et al. Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro[J]. Cellular and Molecular Neurobiology,2014,34(1):101−111. doi: 10.1007/s10571-013-9991-y
[19] SONG X, QIN Y G, YIN Y, et al. Identification and behavioral assays of alarm pheromone in the Vetch Aphid Megoura viciae[J]. Journal of Chemical Ecology, 2021, 47(8-9): 740-746.
[20] 贾殿勇, 高世庆, 段鹏飞, 等. [反]-β-法尼烯合成酶基因在植物抗蚜分子育种中的应用[J]. 生物工程学报,2018,34(1):12−23. [JIA Dianyong, GAO Shiqing, DUAN Pengfei, et al. Metabolic engineering of (E)-β-farnesene synthase genes for aphid-resistant genetically modified plants[J]. Chinese Journal of Biotechnology,2018,34(1):12−23. JIA Dianyong, GAO Shiqing, DUAN Pengfei, et al. Metabolic engineering of (E)-β-farnesene synthase genes for aphid-resistant genetically modified plants[J]. Chinese Journal of Biotechnology, 2018, 34(1): 12-23.
[21] WANG G P, YU X D, FAN J, et al. Expressing an (E)-β-farnesene synthase in the chloroplast of tobacco affects the preference of green peach aphid and its parasitoid[J]. Journal of Integrative Plant Biology,2015,57(9):770−782. doi: 10.1111/jipb.12319
[22] HAN N R, MOON P D, RYU K J, et al. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation[J]. Clinical and Experimental Pharmacology and Physiology,2017,44(2):257−265. doi: 10.1111/1440-1681.12698
[23] ACHARYA B, CHAIJAROENKUL W, NA-BANGCHANG K. Therapeutic potential and pharmacological activities of β-eudesmol[J]. Chemical Biology & Drug Design,2021,97(4):984−996.
[24] NARAHARA C, SAEHENG T, CHAIJAROENKUL W, et al. β-Eudesmol induces the expression of apoptosis pathway proteins in cholangiocarcinoma cell lines[J]. Journal of Research in Medical Sciences,2020,25:7−13. doi: 10.4103/jrms.JRMS_291_19
[25] TSHERING G, PIMTONG W, PLENGSURIYAKARN T, et al. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos[J]. Comparative Biochemistry and Physiology C-toxicology & Pharmacology,2021,243:1−7.
[26] GUO W, LIU S, JU X, et al. The antitumor effect of hinesol, extract from Atractylodes lancea (Thunb.) DC. by proliferation, inhibition, and apoptosis induction via MEK/ERK and NF-κB pathway in non-small cell lung cancer cell lines A549 and NCI-H1299[J]. Journal of Cellular Biochemistry,2019,120(11):18600−18607. doi: 10.1002/jcb.28696
[27] MASUDA Y, KADOKURA T, ISHII M, et al. Hinesol, a compound isolated from the essential oils of Atractylodes lancea rhizome, inhibits cell growth and induces apoptosis in human leukemia HL-60 cells[J]. Journal of Natural Medicines,2015,69(3):332−339. doi: 10.1007/s11418-015-0897-5
[28] 田震, 徐亚元, 李大婧, 等. 基于SPME-GC-MS分析不同干燥方式对香葱挥发油成分的影响[J]. 食品工业科技,2021,42(4):232−244. [TIAN Zhen, XU Yayuan, LI Dajing, et al. Analysis of volatile components in dried chives by SPME-GC-MS[J]. Science and Technology of Food Industry,2021,42(4):232−244. TIAN Zhen, XU Yayuan, LI Dajing, et al. Analysis of volatile components in dried chives by SPME-GC-MS[J]. Science and Technology of Food Industry, 2021, 42(4): 232-244.
[29] 郑文迪, 关倩倩, 刘长根, 等. 基于GC-MS法对比广西地区酸菜和酸笋风味的差异[J]. 食品与发酵工业,2020,46(4):253−257. [ZHENG Wendi, GUAN Qianqian, LIU Changgen, et al. Comparison of flavors of Suancai and Suansun in Guangxi based on GC-MS[J]. Food and Fermentation Industries,2020,46(4):253−257. ZHENG Wendi, GUAN Qianqian, LIU Changgen, et al. Comparison of flavors of Suancai and Suansun in Guangxi based on GC-MS[J]. Food and Fermentation Industries, 2020, 46(4): 253-257.
[30] 滕中秋, 郝庆秀, 金艳, 等. 基于HS-SPME-GC-MS的不同产地彝药满山香挥发性成分比较研究[J]. 中国中药杂志,2018,43(15):3216−3222. [TENG Zhongqiu, HAO Qingxiu, JIN Yan, et al. Comparison of volatile components in Schisandra propinqua from different habitats by HS-SPME-GC-MS[J]. China Journal of Chinese Materia Medica,2018,43(15):3216−3222. TENG Zhongqiu, HAO Qingxiu, JIN Yan, et al. Comparison of volatile components in Schisandra propinqua from different habitats by HS-SPME-GC-MS[J]. China Journal of Chinese Materia Medica, 2018, 43(15): 3216-3222.





 下载:
下载:

 下载:
下载:
