Research Progress of Immunochromatographic Strip Technique in Food Safety
-
摘要: 免疫层析试纸条技术(Immunochromatographic Test Strip,ICTS)结合了色谱分析的分离能力和免疫分析的特异性,具有操作简单,检测快速以及价格低廉的特点,已成为食品安全快速检测领域研究的热点。传统的ICTS是以胶体金作为信号标记材料,但是胶体金试纸条检测灵敏度较低,只适用于定性和半定量检测。为了提升试纸条的检测性能,研究者做了大量的努力。本论文介绍了传统的胶体金试纸条的检测基本原理,并对近年来开发的试纸条检测新技术进行综述,同时也提出该项技术目前所存在的局限性并提出了未来的发展方向,以期为试纸条的进一步开发利用提供一定的文献支持。Abstract: Immunochromatographic test strip (ICTS) combines the separation ability of chromatography with the specificity of conventional immunoassays. It has the characteristics of simple operation, quick detection and low price, which has been a hot spot in the field of rapid detection of food safety. Colloidal gold was employed as the signal marking material in the traditional ICTS. However, ICTS based traditional colloidal gold show relatively low detection sensitivity, which only can meet qualitative and semi-quantitative detection. In order to improve the detection performance of the test strip, researchers have made significant efforts. In this paper, the basic principle of the traditional colloidal gold-based ICTS was introduced and the latest research progress of the ICTS were summarized. In addition, the current limitations and the development direction of immunochromatographic technique was given. This review is expected to provide the bibliographic support for the further development and utilization of test strips.
-
Keywords:
- immunochromatographic /
- test Strip /
- detection technology /
- food safety
-
食品安全是指食品无毒、无害,符合应当有的营养要求,对人体健康不造成任何急性、亚急性或者慢性危害。随着食品工业的发展,不法商贩为了谋取更大的利润,向食品中添加非法添加物,与此同时由食源性致病菌造成的食品污染事件频频爆发,农产品中的农药、兽药残留超标问题屡禁不止。国内外的食品安全恶性事件接连不断的发生,食品安全问题已经成为全球性的大问题。因此,发展食品安全快速检测技术对于有害物质的分析尤为重要。
ICTS结合免疫技术和色谱层析技术,作为食品安全快速检测传感器的一种,具有操作简单,检测快速,成本低廉等特点,这些优点也使得试纸条技术对于致病菌[1]、激素残留[2]、细菌毒素[3]、重金属[4]以及农兽药残留[5]等有毒有害物质的检测越来越广泛。传统的免疫层析试纸条主要以胶体金作为标记物,通过金颗粒在检测线处的聚集对待检目标物进行定性或者半定量检测,这种检测方法检测灵敏度较低,同时最早的试纸条只能用来检测单种目标物。为了提升传统试纸条的检测性能,众多研究者做了巨大的努力。本论文主要对近年来试纸条的检测新技术进行综述,以期为试纸条的进一步开发利用提供借鉴。
1. 传统的免疫层析试纸条的检测原理
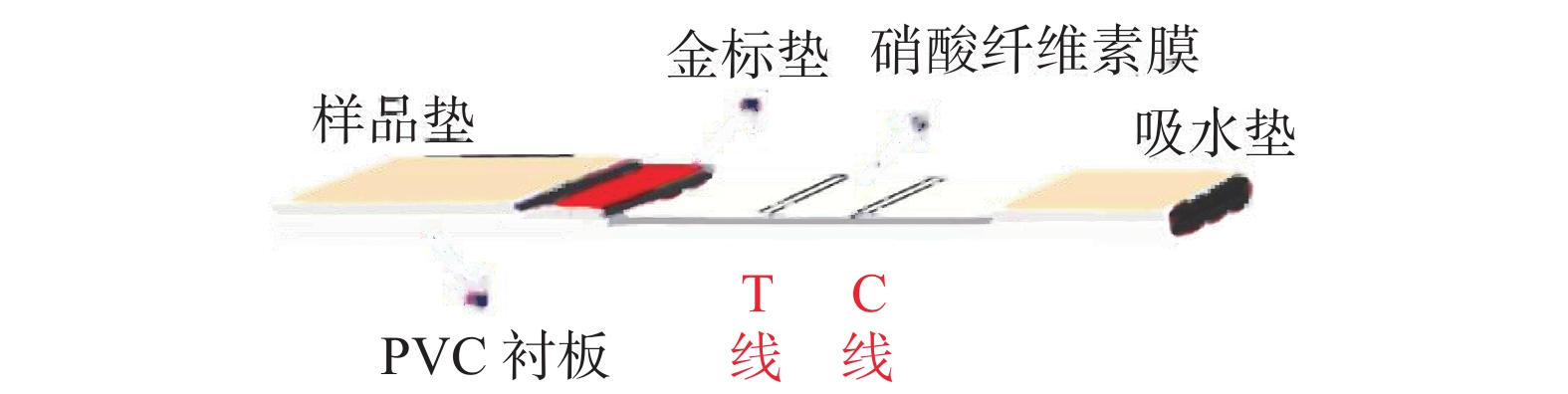
传统的免疫层析试纸条主要由样品垫、金标垫、硝酸纤维素膜、吸水垫以及聚氯乙烯(Polyvinylchloride,PVC)衬板五部分组成,其相互交叠顺序如图1所示。 同时在硝酸纤维素膜上包被两条线,检测线(test line,T线)和质控线(control line,C线)。根据待检测物质分子量的大小可以分为双抗体夹心法和竞争抑制法。
1.1 双抗体夹心法
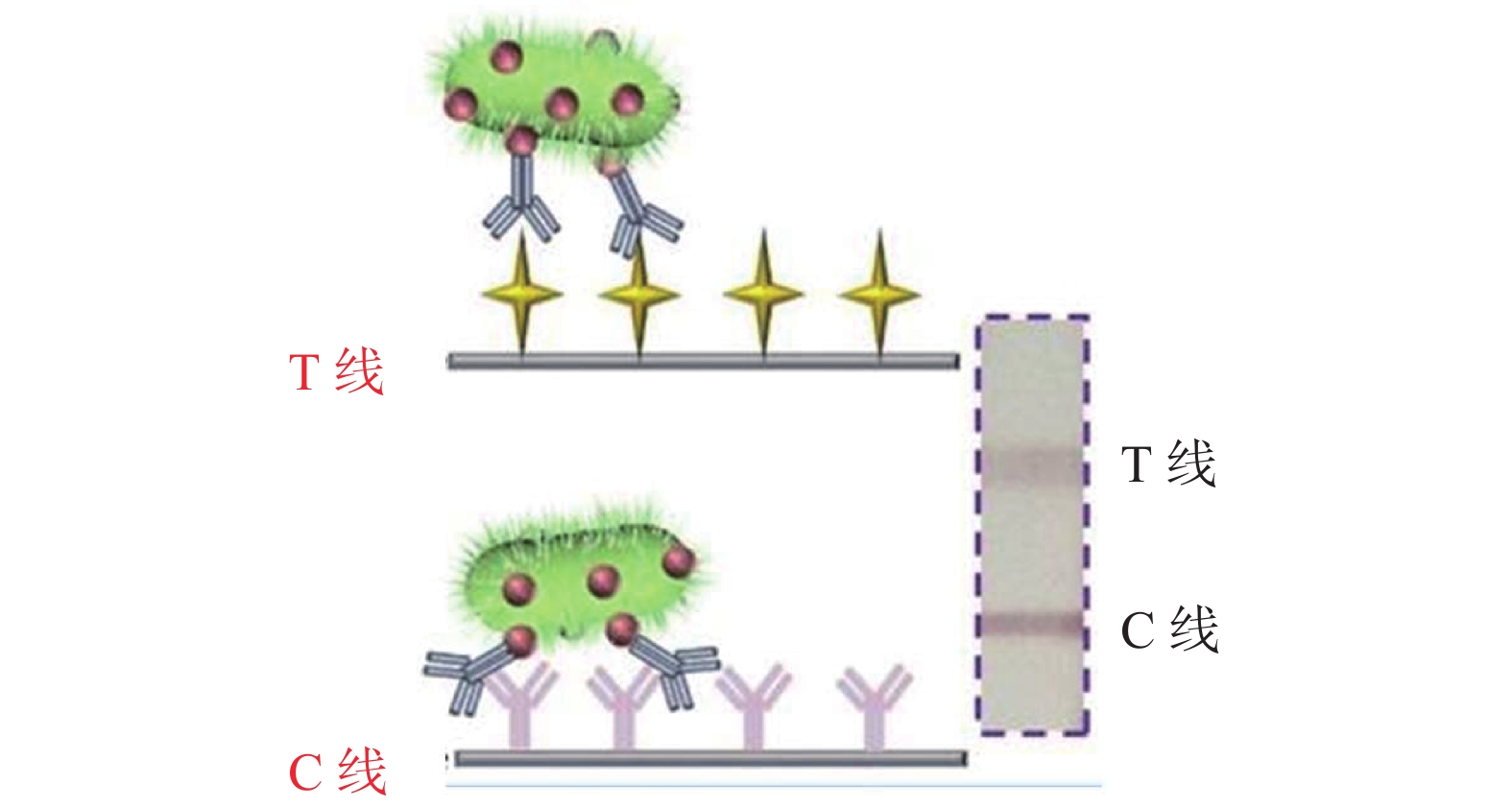
双抗体夹心法主要用来检测具有多个抗原表位的大分子物质和颗粒性抗原(细菌、病毒和蛋白质等),当样品溶液滴加到样品垫时,样品通过毛细管虹吸作用和金标垫处的金标抗体结合形成待检抗原-金标抗体复合物,随着免疫层析的继续进行,该复合物会和T线处的捕获抗体结合,形成捕获抗体-待检抗原-金标抗体复合物的夹心结构。未结合的金标抗体和待检抗原-金标抗体复合物会和C线处的羊抗鼠IgG结合。在双抗体夹心法中T线处的信号颜色会随着待检物的增多而增强。
1.2 竞争抑制法
竞争抑制法主要用来检测小分子物质(细菌毒素、农兽药残留以及激素等),这种方法在T线处包被的是捕获抗原,当把带有小分子的待检溶液滴加到样品垫时,待检小分子随着层析的作用会和金标抗体结合形成待检小分子-金标抗体复合物,该复合物层析到检测线处时,待检小分子和捕获抗原对于金标抗体的特异性位点存在竞争作用,使得只有没和待检小分子结合的金标抗体才可以和T线处的捕获抗原结合,形成捕获抗原-金标抗体复合物。所以竞争法中小分子浓度越高,检测线处的信号强度越弱,并且抗体使用量越少,检测灵敏度也会越高。
2. 免疫层析试纸条检测技术发展方向
2.1 开发新型纳米标记材料
纳米材料是指三维空间尺寸至少有一维尺寸在1~100 nm范围内的超微粒子,由于其较大的表面积、独特的光学特性、表面带电性以及较好的生物相容性,作为免疫层析试纸条的标记物得到了广泛的应用。基于胶体金试纸条的众多检测弊端,为了提升试纸条的检测性能,近年来研究人员对试纸条的纳米标记材料进行了不断的探索。目前应用于试纸条的纳米材料如表1所示。
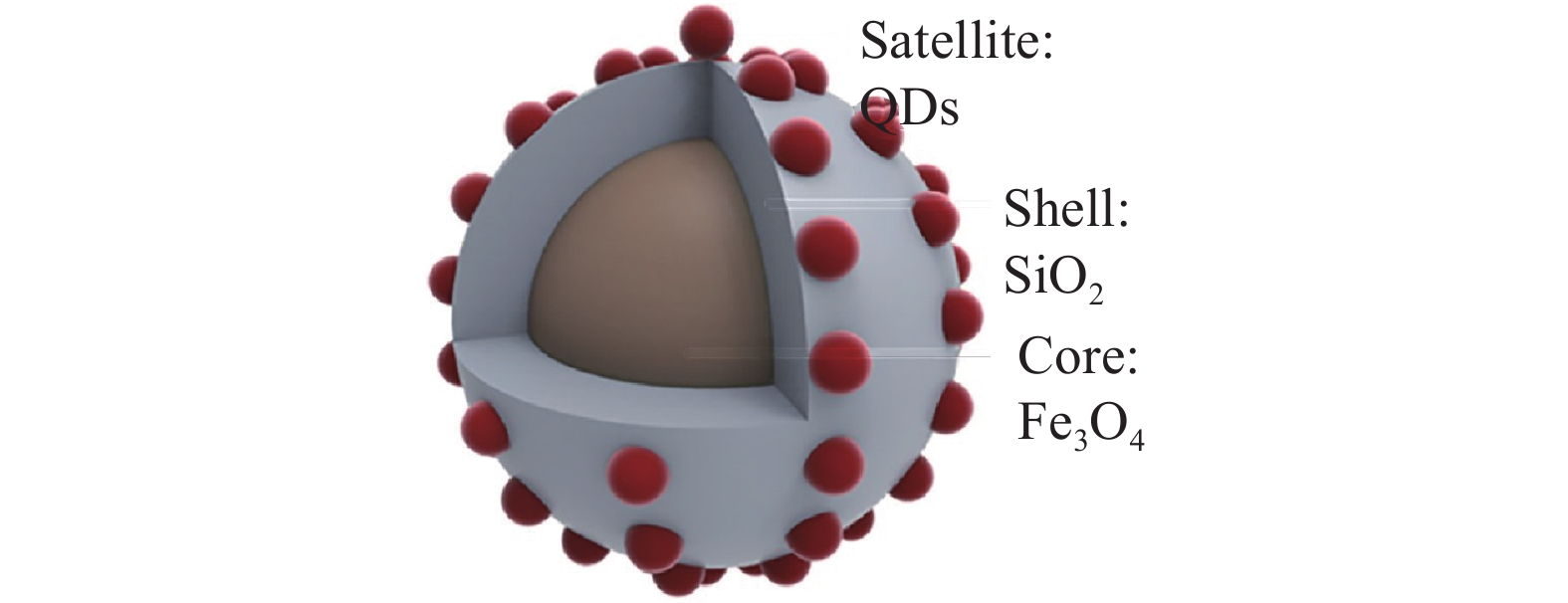
表 1 在免疫层析试纸条技术中应用的纳米颗粒Table 1. Application of nanoparticles in ICTS纳米材料 检测目标物 LOD 性能提高倍数 参考文献 乳胶微球 布鲁氏菌 40 IU·mL−1 − [6] Eu纳米材料 吡虫啉 0.02 ng·mL−1 没有提高 [7] 黄曲霉毒素B1 0.1 µg·kg−1 − [8] 普鲁士蓝纳米颗粒 克伦特罗 1 ng·mL−1 5倍 [9] 核壳二氧化硅纳米粒子 大肠杆菌O157:H7 4.5×10 cfu·mL−1 − [10] 磁性纳米材料 磺胺二甲嘧啶 0.026 ng·mL−1 − [11] 李斯特菌 104 cfu·mL−1 10倍 [12] 硝基呋喃代谢物 0.1 µg·L−1 − [13] 量子点 伏马毒素 2.8 µg·L−1 − [14] 呋喃唑酮 0.1 µg·L−1 − [15] 硅烷化的量子点 玉米赤霉烯酮 40 µg·kg−1 − [16] 上转化颗粒(UCP) 黄曲霉毒素B1 0.03 ng·mL−1 − [17] 碳纳米管 汞粒子 0.05 µg·L−1 10倍 [18] 氧化石墨烯 黄曲霉毒素B1 0.3 ng·mL−1 − [19] 空心金-银纳米材料 克伦特罗 2 µg·L−1 − [20] 铂-金双金属纳米材料 大肠杆菌O157:H7 102 cfu·mL−1 1000倍 [21] g-C3N4/BiFeO3纳米复合材料 毒死蜱和西维因 0.033 ng·mL−1 − [22] Pt-Ni(OH)2纳米片 甲氰菊酯 0.24 ng·mL−1 − [23] 聚多巴胺纳米球 呋喃唑酮 3.5 ng·mL−1 − [24] 二硫化钼纳米片 四环素 0.023 ng·mL−1 17倍 [25] 氧化钴纳米粒子 呋喃唑酮 0.4 ng·mL−1 3倍 [26] 二氧化锰纳米花 毒死蜱 0.033 ng·mL−1 − [27] 磁-金纳米复合材料 赭曲霉毒素A 0.094 ng·mL−1 − [28] 纳米金@多巴胺 玉米赤霉烯酮 7.4 pg·mL−1 10倍 [29] 在免疫层析技术中,单个纳米材料表现出的信号强度有限,为了增强试纸条的灵敏度,研究者试图将单个纳米材料富集起来,材料信号的富集提高了试纸条标记信号的发光强度[30]。Xu等[31]以硅纳米棒作为载体,将纳米金颗粒修饰到硅纳米棒上,纳米金颗粒在硅纳米棒上的富集增强了试纸条的信号强度,这种方法的检测灵敏度比常规胶体金免疫层析试纸条高50倍。Huang等[32]使用四氧化三铁作为核,二氧化硅为壳的核壳纳米材料作为富集量子点的载体进而合成Fe3O4@SiO2@QDs纳米材料(图2),用这种方法检测猪尿中的盐酸克伦特罗,检测灵敏度为0.22 ng·mL−1,比常规胶体金免疫层析试纸条灵敏度高4倍。Zhang等[33]开发了以硅纳米材料为核量子点为壳的纳米材料,该材料以带正点的聚乙烯亚胺作为中间层,使得许多羧基功能化的量子点富集到硅纳米材料表面,使其具有较好的发光性能。用该纳米材料检测鼠伤寒沙门氏菌,检测限为5×102 cfu· mL−1。也有研究者将菌体作为纳米材料的信号富集载体,例如Huang等[34]将纳米金颗粒富集在细菌上,以此作为试纸条的信号标签来检测食品中的克伦特罗,对克伦特罗的检测限为0.1 ng mL−1,灵敏度与传统的胶体金试纸条相比提高了20倍。Bu等[35]利用微生物构建了一种新型的试纸条信号载体,利用纳米金颗粒功能化酵母菌和乳酸杆菌,实现富集金纳米颗粒的作用进而放大检测信号(图3),用该试纸条检测食品中的赭曲霉毒素A(ochratoxin,OTA),检测限为0.1 ng·mL−1,与传统的胶体金试纸条相比灵敏度可以提高8倍。大量的纳米材料聚集在一起提高了试纸条的检测灵敏度,灵敏度的提高降低了试纸条对有毒有害物质检测的检测限,对食品安全检测的意义尤为重要。
2.2 纳米材料标记羊抗鼠抗体法
传统的试纸条是利用纳米材料标记单克隆抗体,羊抗鼠抗体只作为质控线上的抗体用来判定试纸条的有效性。但是近年来研究者开始探索将纳米材料标记羊抗鼠抗体来提高试纸条的检测灵敏度。
Yao等[36]使用羧基化的磁性纳米粒子标记羊抗鼠抗体(magnetic nanoparticles-goat anti mouse antibody,MNPs-GAMA),并将其和未标记的单克隆抗体一并加入待检溶液中,用于检测食品中的β-雌二醇(17β-estradiol,E2),当检测体系中不存在E2时,检测溶液中会形成单克隆抗体-GAMA-MNPs复合物,该复合物会随着免疫层析的作用和检测线处E2-BSA相结合,形成E2-BSA-单克隆抗体-GAMA-MNPs复合物,使得检测线处呈现MNPs的黄颜色。当检测体系中存在E2时,E2和E2-BSA对于单克隆抗体的竞争作用,使得检测线处颜色变浅。用该试纸条检测食品中的E2,研究发现和用纳米材料标记单克隆抗体的传统方法相比,检测灵敏度提高了5倍。同样的原理,Majdinasab等[37]利用荧光铕纳米粒子标记羊抗鼠抗体,用未标记的单克隆抗体捕获待检溶液中的OTA,这种方法对于OTA的检测限可以低至0.4 pg·mL−1,检测限降低了100倍。
这种方法引起灵敏度提高的原因为如下两个方面:第一,由于一个单克隆抗体可以捕获多个MNPs-GAMA,磁性纳米粒子的聚集致使信号强度增强;第二,由于在溶液中未标记的单克隆抗体可以和目标分析物更充分的结合,使得单克隆抗体的用量减少,对于检测小分子的竞争法试纸条来说,单克隆抗体的减少会引发待检小分子和小分子-BSA之间对于单抗更激烈的竞争进而提高试纸条检测的灵敏度。
2.3 待检目标物携带信号法
传统的免疫层析试纸条是用纳米材料标记单克隆抗体,用标记有信号的抗体检测待检物质。而近年来,有研究者报道了将待检测的物质结合上信号而不标记单克隆抗体。
Wang等[38]开发了一种表面带正电的富氮碳纳米材料(positively charged nitrogen-rich carbon,pNC),以这种材料吸附沙门氏菌,同时也将其作为一种显色的信号标签,将单克隆抗体包被在检测线上。当待检体系中有沙门氏菌时,将pNC和待检溶液混合,pNC在静电作用和氢键的作用下捕获目标菌,最后在免疫层析的作用下被检测线上的特异性单克隆抗体捕获,形成pNC-沙门氏菌-抗体的夹心结构。该试纸条对沙门氏菌的检测限为102 cfu·mL−1。Bu等[39]将广谱的抗生素氨苄青霉素(ampicillin, Amp)引入了免疫层析试纸条技术中,将Amp作为抗体的替代物和目标菌结合,利用羧基化的磁性纳米颗粒(magnetite nanoparticles,MNPs)标记Amp,使得形成的MNPs-Amp复合物,该复合物在待检溶液中具有结合,分离和富集目标菌的能力。同时MNPs自带的黄色可以作为一种试纸条的识别信号。该方法的特异性由包被在检测线上的特异性单克隆抗体识别。试纸条对食品中肠炎沙门氏菌的检测范围可以达到102~103 cfu·mL−1。同时Bu等[40]将革兰氏染色的方法和免疫层析试纸条技术相结合,当待检溶液中存在目标菌时,目标菌可以通过一步染色法和结晶紫结合,结晶紫的紫色也可以作为试纸条的识别信号。包被于检测线上的高特异性的单克隆抗体可以保证对目标菌检测的特异性。这种方法可以在11 min内完成对沙门氏菌的检测,检测限可以达到80 cfu mL−1。Wang等[41]利用甘露糖和细菌鞭毛中的FimH蛋白特异性的识别机制,把甘露糖组装到普鲁士蓝纳米颗粒上用来检测大肠杆菌O157:H7,当样品中存在大肠杆菌时,普鲁士蓝纳米颗粒上的甘露糖会和大肠杆菌相结合,随着免疫层析的进行,大肠杆菌-普鲁士蓝纳米颗粒复合物会和T线处的单克隆抗体结合。这种方法对于大肠杆菌的定量检测范围为102~108 cfu·mL−1。
对于致病菌的检测,传统的试纸条通常采用双抗体夹心方法,需要可以和致病菌不同位点结合的一对特异性抗体,而该方法仅需要一种抗体包被于检测线,用于目标菌的特异性识别,最终使得检测成本大大降低。
2.4 染色法
传统的试纸条信号强度较弱,为了提高试纸条的检测信号,研究者试图通过染色的方法使得检测信号进一步增强。Huang等[42]开发了一种铂染色的方法,这种方法首先利用胶体金试纸条对待检目标物进行识别和捕获进而实现定性和半定量检测,紧接着把检测区域剪下来进行染色,在硝酸银和对苯二酚存在的情况下,会在检测区域的纳米金上包裹银壳(Au@AgNPs),接着在H2PtCl6和抗坏血酸溶液中,检测区域的Au@AgNPs上包裹铂壳最终会形成Au@AgPtNPs。经过一系列的染色,试纸条的检测灵敏度大幅提高。银染色法是在银盐溶液和对苯二酚存在的条件下,银离子被胶体金试纸条上的金纳米颗粒催化成金属银,进而沉积在纳米金颗粒表面,不仅使得粒子尺寸变大,也会形成更容易区分的黑色检测区,最终也提高检测灵敏度[43-44]。Yu等[45]用银染色法对玉米样品中的伏马毒素B1(fumonisin B1, FB1)和脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)进行检测,在最优条件下对它们的检测限分别为2.0和40 ng mL−1。和传统的胶体金试纸条相比,灵敏度最少提高2倍。金染色法是以氯金酸(HAuCl4)和盐酸羟胺(NH2OH·HCl)作为增强试剂,在胶体金试纸条检测完毕后,通过滴加该增强试剂,胶体金试纸条上的金纳米颗粒会催化HAuCl4和NH2OH·HCl形成新的金颗粒,并覆盖到原始的纳米金颗粒上使得检测区域颜色变深,进而提高了检测灵敏度[46]。Bu等[47]利用金染色法对牛奶中的肠炎沙门氏菌进行检测,检测限为104 cfu·mL−1,相比于传统的胶体金试纸条,检测灵敏度提高了100倍。
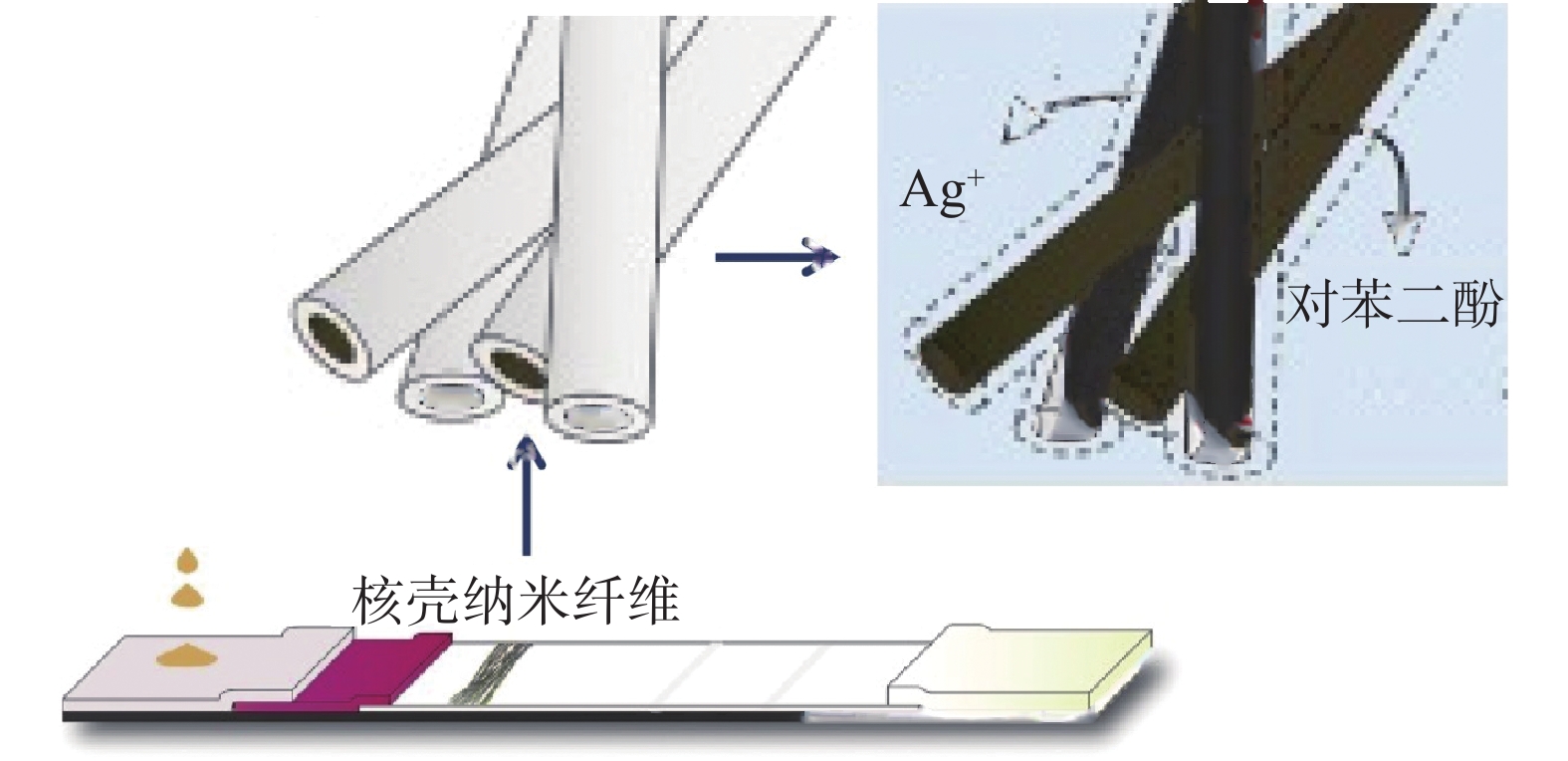
上述方法中需要额外的步骤对试纸条进行染色,最终使得检测时间延长。Kim等[48]在试纸条中引入核-壳纳米纤维,该纳米纤维的壳由水溶性的聚合物组成,核心分别由银盐溶液试剂和对苯二酚组成,这些纳米纤维通过静电纺丝技术而直接沉积到硝酸纤维素膜T线的前端。随着样品的加入,可溶性的纳米纤维中的银盐溶液试剂和对苯二酚会随着免疫层析的进行而溶解出来,进而在检测线处的金纳米颗粒上沉积上银颗粒,最终使得T线处的颜色变黑。用该种方法可使得检测限提高10倍,同时不会增加检测的时间(图4)。
2.5 双结合垫法
传统的试纸条使用单个结合垫,结合垫上结合的是纳米材料标记的抗待检目标物的单克隆抗体(纳米材料-Ab1),这种方法表现出的信号强度较弱,为了提高试纸条的检测性能,研究者开始突破试纸条传统结构的限制,开发了带有双结合垫的免疫层析试纸条。在双结合垫方法中,一种方法是在第二个结合垫上结合纳米材料标记的抗牛血清白蛋白(Bovine Serum Albumin,BSA)的单克隆抗体(纳米材料-Ab2),由于在纳米材料标记单克隆抗体的过程中,会用到BSA来封闭纳米材料表面的剩余位点,所以纳米材料-Ab2会和纳米材料-Ab1上的BSA结合,形成纳米材料-Ab2-纳米材料-Ab1复合物,进而实现了纳米材料的聚集,提高了信号强度[49-50]。
第二种方法是在第二个结合垫上结合纳米材料标记的羊抗鼠抗体(纳米材料-GAMA),利用GAMA可以和待检目标物的单克隆抗体结合的原理,随着免疫层析的进行会形成纳米材料-GAMA-Ab1-纳米材料复合物,纳米材料的聚集也提高了检测信号的强度[51]。
第三种方法是引入生物素(biotin)-链霉亲和素(streptavidin)系统,在第一个结合垫上结合纳米材料标记生物素化的单克隆抗体(纳米材料-BAb),第二个结合垫上结合纳米材料标记的链霉亲和素(纳米材料-Sa),随着免疫层析的进行会形成纳米材料-BAb-Sa-纳米材料复合物,也实现了纳米材料的聚集进而提高了检测的灵敏度[52]。
2.6 多元检测法
传统的免疫层析试纸条主要用来检测单一的目标物,为了提高试纸条的检测效率,研究人员设计了可以同时检测多种目标物质的试纸条。
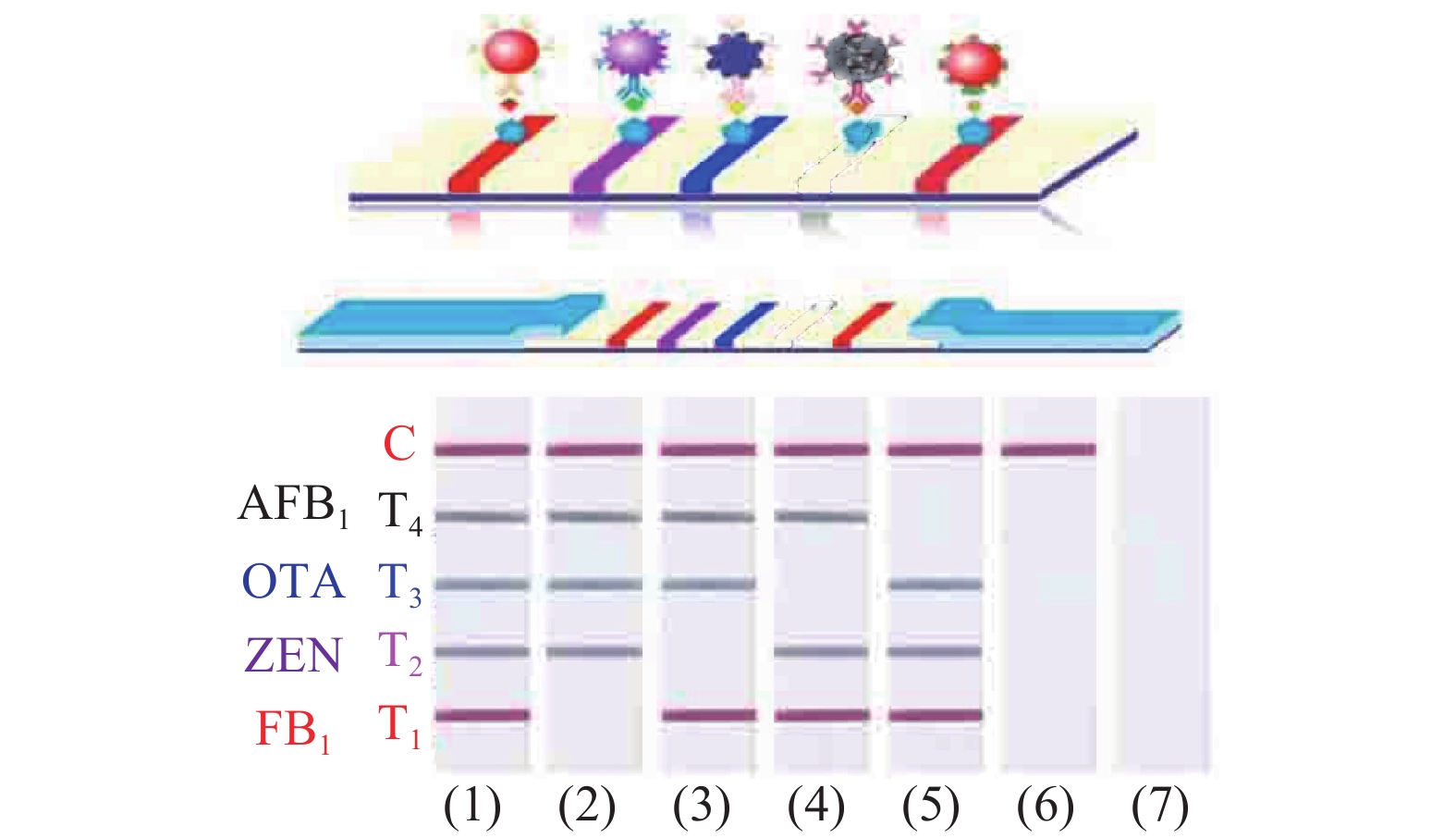
为了实现多元检测,一种方法是利用单一的纳米颗粒标记多种抗待检目标物质的抗体,同时在试纸条上面设计多条检测线[53]。Kong等[54]开发了基于金纳米颗粒的免疫层析试纸条用来检测谷类食物样品中的20多种霉菌毒素,属于玉米赤霉烯酮类、脱氧雪腐镰刀菌烯醇类、T-2毒素类、黄曲霉毒素类和伏马毒素类五个大类。整个检测过程共耗时20 min,大大提高了检测速度。第二种方法是采用多种颜色的纳米材料对多种目标物质进行检测,每种目标物质对应的检测线的颜色均不同,便于更好地区分待检物质[55]。Wu等[56]合成四种颜色的金纳米材料(图5),分别为红色的金纳米球(gold nanospheres,AuNSs),紫色的金纳米仙人掌(gold nanocacti,AuNCs),蓝色的金纳米花(gold nanoflowers,AuNFs)和黑色的分枝金等离子体黑体(hyperbranched Au plasmonic blackbodies,AuPBs),用这四种金纳米材料分别对FB1,玉米赤霉烯酮(zearalenone,ZEN),OTA,和黄曲霉毒素B1(aflatoxin B1, AFB1)进行同时检测,检测限分别为3.27、0.70、0.10和0.06 ng·mL−1。Taranova等[57]利用在625、585和525 nm波长处具有最大发射峰而呈现出红色、黄色和绿色的多色量子点开发了一种“交通信号灯”的免疫层析试纸条,利用该试纸条对牛奶中氧氟沙星(ofloxacin,OFL)、氯霉素(chloramphenicol,CAP)和链霉素(streptomycin,STM)三种抗生素同时进行检测。对这三种目标物质的检测限为0.3, 0.12和0.2 ng mL−1。在使用相同抗体的条件下,检测限比酶联免疫吸附方法低80~200倍。
2.7 物理阻碍作用法
在免疫层析技术中通过减小纸张的空隙和减慢液体流动的速度可以提高试纸条的检测性能。Quesada-González等[58]将纤维素纳米纤维(cellulose nanofbers,CNF)喷涂到免疫层析试纸条的检测区域,CNF浸入到硝酸纤维素膜的空隙内部,进而减小了检测区域膜的孔径。这种修饰使得检测区的抗体更接近试纸条表面,最终使得试纸条表面金纳米颗粒的结合数量增多,使得检测灵敏度提高了36.6%(图6)。Wu等[59]在试纸条下面放置磁铁以提供外加磁场,该磁场会和试纸条上的磁性纳米标记材料Fe3O4@Au相互作用,减慢了免疫试剂的流动速度同时增加了试剂之间的相互作用时间,最终使得检测灵敏度提高。Choi等[60]通过在试纸条上增加玻璃纤维分流垫以及在试纸条上设置聚二甲基硅氧烷障碍点来减慢免疫试剂在试纸条上的流动速度,同时也增加了试剂之间的反应时间,用该方法检测乙型肝炎病毒,使得检测灵敏度提高了10倍。
2.8 淬灭荧光法
Morales-Narváez等[61]使用量子点代替传统试纸条质控线上的羊抗鼠抗体,在检测线处包被量子点标记的检测抗体(Ab-QDs),同时结合氧化石墨烯对于量子点的淬灭能力对大肠杆菌进行检测。在有目标检测菌大肠杆菌存在的情况下,大肠杆菌可以和检测线处的Ab-QDs结合形成Ab-QDs-大肠杆菌复合物,紧接着在试纸条上滴加氧化石墨烯,大肠杆菌的存在阻碍了量子点和石墨烯之间的能量转移,此时石墨烯不能淬灭量子点的荧光。而在没有目标菌存在的情况下,检测线处Ab-QDs的荧光可以被石墨烯淬灭。这种方法对瓶装水和牛奶中的大肠杆菌的检测限为100 cfu·mL−1。Hassan等[62]用该种方法对牛肉馅和河水中的大肠杆菌O157:H7进行检测,对牛肉馅的检测限为178 cfu·g−1,河水的检测限为133 cfu·mL−1。检测灵敏度相比传统的试纸条提高了1000倍,同时量子点代替羊抗鼠抗体也使得检测成本降低了60%。
2.9 多种模式读取信号法
传统的胶体金试纸条主要是根据试纸条上纳米标记材料的颜色进行比色来判断结果,近年来,研究者开发出了既能肉眼比色检测也能使得试纸条可以通过一定的化学反应进行定量分析结果的试纸条。
Ouyang等[63-64]通过使用鲁米诺还原氯金酸制备出了鲁米诺还原的金纳米粒子(Luminol-reduced gold nanoparticles,LuReGNPs),该方法以LuReGNPs在试纸条上的红颜色作为定性和半定量的判定结果,在免疫层析过程完成后,将检测区域剪下来放入微孔板的孔内,在孔内加入辣根过氧化物酶(HRP)和过氧化氢,最终可收集到另外的化学发光信号,此信号可以作为定量检测的依据。用该方法检测农药残留,对甲基对硫磷和甲氰菊酯的检测限分别为0.17 ng·mL−1和0.10 ng·mL−1。同时Ouyang等[27]以MnO2纳米花作为试纸条的信号标签,检测线处表现出MnO2纳米花的棕色可以作为定性和半定量的检测信号,同时MnO2纳米花对鲁米诺-H2O2化学发光体系具有显著的影响,也可以通过剪下检测区域来收集化学发光信号,用该方法对毒死蜱的检测限为0.033 ng·mL−1。同理Ouyang等[22]也开发了g-C3N4/BiFeO3纳米符合材料,通过双信号读出对毒死蜱和西维因进行检测,对它们的检测限为0.033 ng·mL−1。Wang等[41]使用普鲁士蓝纳米颗粒开发了三种读出模式的试纸条,分别为肉眼定性检测,软件分析检测线信号强度进行定量检测,以及利用普鲁士蓝纳米颗粒的过氧化物酶活性催化四甲基联苯胺(TMB)显色的比色信号进行定量检测。
3. 结语
ICTS经过多年的发展已经较为成熟,在众多领域已经实现商品化应用。本文简要总结了近年来该技术相比于传统的胶体金试纸条的改进方向,尽管ICTS性能有很大的提升,但在实际应用方面仍然存在很多的问题。目前,主要面临的问题及发展的趋势简述如下:(1)试纸条技术中对于有害物质的特异性分析依赖于单克隆抗体,但是单克隆抗体制备速度较慢,需要消耗大量的人力物力,快速地开发廉价、高亲和力的单克隆抗体仍然是目前的一大挑战,随着纳米抗体在免疫分析中的应用越来越广泛,未来可以考虑将纳米抗体应用于ICTS[65-66]。(2)有些纳米标记材料的合成需要严苛的化学反应条件,同时合成时间较长,开发可以在温和条件下快速合成的纳米材料对ICTS具有重要的作用。(3)检测样品的复杂成分会影响试纸条的检测灵敏度,降低检测基质对于试纸条的检测性能的影响对检测性能的提高具有重要的意义。(4)ICTS准确的检测信号读出依赖于信号读出设备,开发简易便携便宜的小型仪器也是未来的发展趋势。
-
表 1 在免疫层析试纸条技术中应用的纳米颗粒
Table 1 Application of nanoparticles in ICTS
纳米材料 检测目标物 LOD 性能提高倍数 参考文献 乳胶微球 布鲁氏菌 40 IU·mL−1 − [6] Eu纳米材料 吡虫啉 0.02 ng·mL−1 没有提高 [7] 黄曲霉毒素B1 0.1 µg·kg−1 − [8] 普鲁士蓝纳米颗粒 克伦特罗 1 ng·mL−1 5倍 [9] 核壳二氧化硅纳米粒子 大肠杆菌O157:H7 4.5×10 cfu·mL−1 − [10] 磁性纳米材料 磺胺二甲嘧啶 0.026 ng·mL−1 − [11] 李斯特菌 104 cfu·mL−1 10倍 [12] 硝基呋喃代谢物 0.1 µg·L−1 − [13] 量子点 伏马毒素 2.8 µg·L−1 − [14] 呋喃唑酮 0.1 µg·L−1 − [15] 硅烷化的量子点 玉米赤霉烯酮 40 µg·kg−1 − [16] 上转化颗粒(UCP) 黄曲霉毒素B1 0.03 ng·mL−1 − [17] 碳纳米管 汞粒子 0.05 µg·L−1 10倍 [18] 氧化石墨烯 黄曲霉毒素B1 0.3 ng·mL−1 − [19] 空心金-银纳米材料 克伦特罗 2 µg·L−1 − [20] 铂-金双金属纳米材料 大肠杆菌O157:H7 102 cfu·mL−1 1000倍 [21] g-C3N4/BiFeO3纳米复合材料 毒死蜱和西维因 0.033 ng·mL−1 − [22] Pt-Ni(OH)2纳米片 甲氰菊酯 0.24 ng·mL−1 − [23] 聚多巴胺纳米球 呋喃唑酮 3.5 ng·mL−1 − [24] 二硫化钼纳米片 四环素 0.023 ng·mL−1 17倍 [25] 氧化钴纳米粒子 呋喃唑酮 0.4 ng·mL−1 3倍 [26] 二氧化锰纳米花 毒死蜱 0.033 ng·mL−1 − [27] 磁-金纳米复合材料 赭曲霉毒素A 0.094 ng·mL−1 − [28] 纳米金@多巴胺 玉米赤霉烯酮 7.4 pg·mL−1 10倍 [29] -
[1] Song Chunmei, Liu Cheng, Wu Shuyan, et al. Development of a lateral flow colloidal gold immunoassay strip for the simultaneous detection of Shigella boydii andEscherichia coli O157: H7 in bread, milk and jelly samples[J]. Food Control,2016,59:345−351. doi: 10.1016/j.foodcont.2015.06.012
[2] Yang Xingdong, Wang Fangyu, Song Chunmei, et al. Establishment of a lateral flow colloidal gold immunoassay strip for the rapid detection of estradiol in milk samples[J]. LWT - Food Science and Technology,2015,64(1):88−94. doi: 10.1016/j.lwt.2015.04.022
[3] Chen Yiqiang, Chen Qian, Han Miaomiao, et al. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut[J]. Food Chemistry,2016,213:478−484. doi: 10.1016/j.foodchem.2016.06.116
[4] Liu Xi, Xiang Junjain, Tang Yong, et al. Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples[J]. Analytica Chimica Acta,2012,745:99−105. doi: 10.1016/j.aca.2012.06.029
[5] Wu Yuchen, Guo Shuang, Dong Qing, et al. Development of an immunochromatographic test strip for rapid simultaneous detection of enrofloxacin and ofloxacin in tissue of chicken muscle and pork[J]. Food Analytical Methods,2016,9(10):2807−2813. doi: 10.1007/s12161-016-0474-x
[6] Zhu Mingsong, Jia Yurui, Peng Lizhi, et al. A highly sensitive dual-color lateral flow immunoassay for brucellosis using one-step synthesized latex microspheres[J]. Analytical Methods,2019,11(22):2937−2942. doi: 10.1039/C9AY00944B
[7] Tan Guiyu, Zhao Yajie, Wang Mian, et al. Ultrasensitive quantitation of imidacloprid in vegetables by colloidal gold and time-resolved fluorescent nanobead traced lateral flow immunoassays[J]. Food Chemistry,2020,311:126055. doi: 10.1016/j.foodchem.2019.126055
[8] Wang Du, Zhang Zhaowei, Li Peiwu, et al. Time-Resolved Fluorescent Immunochromatography of Aflatoxin B1 in Soybean Sauce: A Rapid and Sensitive Quantitative Analysis[J]. Sensors,2016,16(7):1094. doi: 10.3390/s16071094
[9] Zhao Bingxin, Huang Qiong, Dou Leina, et al. Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol[J]. Sensors and Actuators B: Chemical,2018,275:223−229. doi: 10.1016/j.snb.2018.08.029
[10] Zhu Chunjie, Zhao Guangying, Dou Wenchao, et al. Core-shell red silica nanoparticles based immunochromatographic assay for detection ofEscherichia coli O157: H7[J]. Analytica Chimica Acta,2018,1038:97−104. doi: 10.1016/j.aca.2018.07.003
[11] Fang Bolong, Hu Song, Wang Chun, et al. Lateral flow immunoassays combining enrichment and colorimetry-fluorescence quantitative detection of sulfamethazine in milk based on trifunctional magnetic nanobeads[J]. Food Control,2019,98:268−273. doi: 10.1016/j.foodcont.2018.11.039
[12] Shi Lei, Wu Feng, Wen Yiming, et al. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay[J]. Analytical and Bioanalytical Chemistry,2015,407(2):529−535. doi: 10.1007/s00216-014-8276-8
[13] Lu Xuewen, Liang xiaoling, Dong Jianghong, et al. Lateral flow biosensor for multiplex detection of nitrofuran metabolites based on functionalized magnetic beads[J]. Analytical and Bioanalytical Chemistry,2016,408(24):6703−6709. doi: 10.1007/s00216-016-9787-2
[14] F Di Nardo, L Anfossi, C Giovannoli, et al. A fluorescent immunochromatographic strip test using Quantum Dots for fumonisins detection[J]. Talanta,2016,150:463−468. doi: 10.1016/j.talanta.2015.12.072
[15] Le Tao, Xie Yong, Zhu Liqian, et al. Rapid and Sensitive Detection of 3-Amino-2-oxazolidinone Using a Quantum Dot-Based Immunochromatographic Fluorescent Biosensor[J]. Journal of Agricultural and Food Chemistry,2016,64(45):8678−8683. doi: 10.1021/acs.jafc.6b03732
[16] Olga A Goryacheva, Chris Guhrenz, Kristian Schneider, et al. Silanized luminescent quantum dots for the simultaneous multicolor lateral flow immunoassay of two mycotoxins[J]. Acs Applied Materials & Interfaces,2020,12(22):24575−24584.
[17] Zhao Yong, Liu Xiao, Wang Xiaochen, et al. Development and evaluation of an up-converting phosphor technology-based lateral flow assay for rapid and quantitative detection of aflatoxin B1 in crops[J]. Talanta,2016,161:297−303. doi: 10.1016/j.talanta.2016.08.058
[18] Yao Li, Teng Jun, Zhu Mengya, et al. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions[J]. Biosensors & Bioelectronics,2016,85:331−336.
[19] Yu Li, Li Peiwu, Ding Xiaoxia, et al. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays[J]. Talanta,2017,165:167−175. doi: 10.1016/j.talanta.2016.12.042
[20] Wang Jingyun, Zhang Lei, Huang Youju, et al. Hollow Au-Ag nanoparticles labeled immunochromatography strip for highly sensitive detection of clenbuterol[J]. Scientific Reports,2017,7:41419. doi: 10.1038/srep41419
[21] Jiang Tao, Song Yang, Wei Tianxiang, et al. Sensitive detection of Escherichia coli O157: H7 using Pt-Au bimetal nanoparticles with peroxidase-like amplification[J]. Biosensors & Bioelectronics,2016,77:687−694.
[22] Ouyang Hui, Tu Xinman, Fu Zhifeng, et al. Colorimetric and chemiluminescent dual-readout immunochromatographic assay for detection of pesticide residues utilizing g-C3N4/BiFeO3 nanocomposites[J]. Biosensors & Bioelectronics,2018,106:43−49.
[23] Cheng Nan, Shi Qiurong, Zhu Chengzhou, et al. Pt-Ni(OH)2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides[J]. Biosensors & Bioelectronics,2019,142:111498.
[24] Liu Sijie, Dou Leina, Yao Xiaoling, et al. Polydopamine nanospheres as high-affinity signal tag towards lateral flow immunoassay for sensitive furazolidone detection[J]. Food Chemistry,2020,315:126310. doi: 10.1016/j.foodchem.2020.126310
[25] Bu Tong, Jia Pei, Sun Xinyu, et al. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples[J]. Sensors and Actuators B: Chemical,2020,320:128440. doi: 10.1016/j.snb.2020.128440
[26] Su Lihong, Wang Lulu, Yao Xiaolin, et al. Small size nanoparticles-Co3O4 based lateral flow immunoassay biosensor for highly sensitive and rapid detection of furazolidone[J]. Talanta,2020,211:120729. doi: 10.1016/j.talanta.2020.120729
[27] Ouyang Hui, Lu Qian, Wang wenwen, et al. Dual-Readout Immunochromatographic Assay by Utilizing MnO2 Nanoflowers as the Unique Colorimetric/Chemiluminescent Probe[J]. Analytical Chemistry,2018,90(8):5147−5152. doi: 10.1021/acs.analchem.7b05247
[28] Hao Liangwen, Chen Jing, Chen Xirui, et al. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice[J]. Food Chemistry,2021,336:127710. doi: 10.1016/j.foodchem.2020.127710
[29] Xu Shaolan, Zhang Ganggang, Fang Bolong, et al. Lateral flow immunoassay based on polydopamine-coated gold nanoparticles for the sensitive detection of zearalenone in maize[J]. ACS Applied Materials & Interfaces,2019,11(34):31283−31290.
[30] Guo L, Shao Yanna, Duan Hong, et al. Magnetic quantum dot nanobead-based fluorescent immunochromatographic assay for the highly sensitive detection of aflatoxin B1 in dark soy sauce[J]. Analytical Chemistry,2019,91(7):4727−4734. doi: 10.1021/acs.analchem.9b00223
[31] Xu Hui, Chen Jiao, Joseph B, et al. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins[J]. Analytical Chemistry,2014,86(15):7351−7359. doi: 10.1021/ac502249f
[32] Huang Zhen, Xiong Zhijuan, Chen Yuan, et al. Sensitive and matrix-tolerant lateral flow immunoassay based on fluorescent magnetic nanobeads for the detection of clenbuterol in swine urine[J]. Journal of Agricultural and Food Chemistry,2019,67(10):3028−3036. doi: 10.1021/acs.jafc.8b06449
[33] Zhang Bo, Yang Xingsheng, Liu Xiaoxian, et al. Polyethyleneimine-interlayered silica-core quantum dot-shell nanocomposites for sensitive detection of Salmonella typhimurium via a lateral flow immunoassay[J]. Rsc Advances,2020,10(5):2483−2489. doi: 10.1039/C9RA09252H
[34] Huang Qiong, Bu Tong, Zhang Wentao, et al. An improved clenbuterol detection by immunochromatographic assay with bacteria@Au composite as signal amplifier[J]. Food Chemistry,2018,262:48−55. doi: 10.1016/j.foodchem.2018.04.085
[35] Bu Tong, Zhang Meng, Sun Xinyu, et al. Gold nanoparticles-functionalized microorganisms assisted construction of immunobiosensor for sensitive detection of ochratoxin A in food samples[J]. Sensors and Actuators B: Chemical,2019,299:126969. doi: 10.1016/j.snb.2019.126969
[36] Yao Xiaolin, Wang Zonghan, Dou Leina, et al. An innovative immunochromatography assay for highly sensitive detection of 17β-estradiol based on an indirect probe strategy[J]. Sensors and Actuators B: Chemical,2019,289:48−55. doi: 10.1016/j.snb.2019.03.078
[37] Marjan Majdinasab, Mohsen Zareian, Zhang Qi, et al. Development of a new format of competitive immunochromatographic assay using secondary antibody-europium nanoparticle conjugates for ultrasensitive and quantitative determination of ochratoxin A[J]. Food Chemistry,2019,275:721−729. doi: 10.1016/j.foodchem.2018.09.112
[38] Wang Zonghan, Yao Xiaolin, Wang Rong, et al. Label-free strip sensor based on surface positively charged nitrogen-rich carbon nanoparticles for rapid detection of Salmonella enteritidis[J]. Biosensor & Bioelectronics, 2019, 132: 360-367.
[39] Bu Tong, Yao Xiaolin, Huang Lunjie, et al. Dual recognition strategy and magnetic enrichment based lateral flow assay toward Salmonella enteritidis detection[J]. Talanta,2020,206:120204. doi: 10.1016/j.talanta.2019.120204
[40] Bu Tong, Huang Qiong, Yan Lingzhi, et al. Applicability of biological dye tracer in strip biosensor for ultrasensitive detection of pathogenic bacteria[J]. Food Chemistry,2019,274:816−821. doi: 10.1016/j.foodchem.2018.09.066
[41] Wang Zonghan, Yao Xiaolin, Zhang Yongzhi, et al. Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157: H7[J]. Food Chemistry,2020,329:127224. doi: 10.1016/j.foodchem.2020.127224
[42] Huang Di, Lin Bingqian, Song Yanling, et al. Staining traditional colloidal gold test strips with pt nanoshell enables quantitative point-of-care testing with simple and portable pressure meter readout[J]. Acs Applied Materials & Interfaces,2019,11(2):1800−1806.
[43] Myriam Oliveira Rodriguez, Lucía Blanco Covian, Agustín Costa Garcia, et al. Silver and gold enhancement methods for lateral flow immunoassays[J]. Talanta,2016,148:272−278. doi: 10.1016/j.talanta.2015.10.068
[44] V G Panferov, I V Safenkova, Y A Varitsev, et al. Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of Ralstonia solanacearum in potato tubers[J]. Talanta,2016,152:521−530. doi: 10.1016/j.talanta.2016.02.050
[45] Yu Qing, Li Heng, Li Chenglong, et al. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol[J]. Food Control,2015,54:347−352. doi: 10.1016/j.foodcont.2015.02.019
[46] Wang Jingyun, Chen Minghui, Sheng Zhichao, et al. Development of colloidal gold immunochromatographic signal-amplifying system for ultrasensitive detection of Escherichia coli O157: H7 in milk[J]. Rsc Advances,2015,5(76):62300−62305. doi: 10.1039/C5RA13279G
[47] Bu Tong, Huang Qiong, Yan Lingzhi, et al. Ultra technically-simple and sensitive detection for Salmonella Enteritidis by immunochromatographic assay based on gold growth[J]. Food Control,2018,84:536−543. doi: 10.1016/j.foodcont.2017.08.036
[48] Wuseok Kim, Sanghee Lee, Sangmin Jeon, et al. Enhanced sensitivity of lateral flow immunoassays by using water-soluble nanofibers and silver-enhancement reactions[J]. Sensors and Actuators B: Chemical,2018,273:1323−1327. doi: 10.1016/j.snb.2018.07.045
[49] Mei Zhanlong, Qu Wei, Deng Yi, et al. One-step signal amplified lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A (BPA) in aqueous samples[J]. Biosensors & Bioelectronics,2013,49:457−461.
[50] Zhong Youhao, Chen Yinji, Yao Li, et al. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening[J]. Microchimica Acta, 2016, 183(6): 1989-1994.
[51] Dou Leina, Zhao Bingxin, Bu Tong, et al. Highly sensitive detection of a small molecule by a paired labels recognition system based lateral flow assay[J]. Analytical and Bioanalytical Chemistry,2018,410(13):3161−3170. doi: 10.1007/s00216-018-1003-0
[52] Fang Qingkui, Wang Limin, Cheng Qi, et al. A bare-eye based one-step signal amplified semiquantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples[J]. Analytica Chimica Acta,2015,881:82−89 (in Chinese). doi: 10.1016/j.aca.2015.04.047
[53] Shao Yanna, Duan Hong, Guo Liang, et al. Quantum dot nanobead-based multiplexed immunochromatographic assay for simultaneous detection of aflatoxin B1 and zearalenone[J]. Analytica Chimica Acta,2018,1025:163−171. doi: 10.1016/j.aca.2018.03.041
[54] Kong Dezhao, Liu Liqiang, Song Shanshan, et al. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins[J]. Nanoscale,2016,8(9):5245−5253. doi: 10.1039/C5NR09171C
[55] Rong Zhen, Bai Zikun, Li Jianing, et al. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen[J]. Biosensors & Bioelectronics,2019,145:111719.
[56] Wu Yuhao, Zhou Yaofeng, Huang Hai, et al. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor[J]. Sensors and Actuators B: Chemical,2020,316:128107. doi: 10.1016/j.snb.2020.128107
[57] N A Taranova, A N Berlina, A V Zherdev, et al. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk[J]. Biosensors & Bioelectronics,2015,63(2):255−261.
[58] Daniel Quesada-González, Christina Stefani, Israel Gonzalez, et al. Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers[J]. Biosensors & Bioelectronics,2019,141:111407.
[59] Wu Zhengzong, He Deyun, Xu Enbo, et al. Rapid detection of β-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification[J]. Food Chemistry,2018,269:375−379. doi: 10.1016/j.foodchem.2018.07.011
[60] Jane Ru Choi, Liu Zhi, Hu Jie, et al. Polydimethylsiloxane-paper hybrid lateral flow assay for highly sensitive point-of-care nucleic acid testing[J]. Analytical Chemistry,2016,88(12):6254−6264. doi: 10.1021/acs.analchem.6b00195
[61] Eden Morales-Narváez, Tina Naghdi, Erhan Zor, et al. Photoluminescent lateral-flow immunoassay revealed by graphene oxide: highly sensitive paper-based pathogen detection[J]. Analytical Chemistry,2015,87(16):8573−8577. doi: 10.1021/acs.analchem.5b02383
[62] Abdelrahim Hussein Abdelazeem Hassan, Jose Francisco Bergua, Eden Morales-Narvaez, et al. Validity of a single antibody-based lateral flow immunoassay depending on graphene oxide for highly sensitive determination of E. coli O157: H7 in minced beef and river water[J]. Food Chemistry,2019,297:124965. doi: 10.1016/j.foodchem.2019.124965
[63] Ouyang Hui, Wang Mengyao, Wang Wenwen, et al. Colorimetric/chemiluminescent immunochromatographic test strip by using luminol-reduced gold nanoparticles as dual-response probes[J]. Sensors and Actuators B: Chemical, 2018, 266: 318-322.
[64] Ouyang Hui, Wang Wenwen, Shu Qi, et al. Novel chemiluminescent immunochromatographic assay using a dual-readout signal probe for multiplexed detection of pesticide residues[J]. Analyst,2018.
[65] He Yixin, Ren Yarong, Guo Bing, et al. Development of a specific nanobody and its application in rapid and selective determination ofSalmonella enteritidis in milk[J]. Food Chemistry,2020,310:125942. doi: 10.1016/j.foodchem.2019.125942
[66] Wang Feng, Li Zhenfeng, Yang Yuanyuan, et al. Chemiluminescent enzyme immunoassay and bioluminescent enzyme immunoassay for tenuazonic acid mycotoxin by exploitation of nanobody and nanobody-nanoluciferase fusion[J]. Analytical Chemistry,2020, 92 :11935–11942.





 下载:
下载:

 下载:
下载:
